[(2-Chlorophenyl)methylene]malononitrile: properties, applications and safety
Oct 18,2023
General Description
[(2-Chlorophenyl)methylene]malononitrile, also known as CS tear gas, is a widely used riot control agent. It is highly effective in dispersing and incapacitating individuals during civil unrest or riots. CS gas causes severe irritation to the eyes, skin, and respiratory system. It is persistent and can remain in the environment for extended periods. However, its use carries significant health risks, particularly for individuals with pre-existing respiratory conditions. CS gas has low lethality potential and is regulated to ensure responsible deployment. It is toxic when inhaled or in contact with the skin, causing irritation and respiratory discomfort. Prolonged exposure to high concentrations can lead to pulmonary edema and severe bronchospasm. CS tear gas is classified as a poison when ingested and is considered a human skin and eye irritant. Limited evidence suggests a link between CS exposure and carcinogenicity. Overall, CS tear gas serves as a critical tool for maintaining public order but requires careful consideration of its potential hazards.
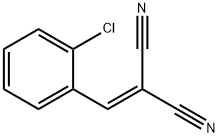
![Figure 1. [(2-Chlorophenyl)methylene]malononitrile.png Figure 1. [(2-Chlorophenyl)methylene]malononitrile.png](/NewsImg/2023-10-01/6383177416056814323387027.jpg)
Figure 1. [(2-Chlorophenyl)methylene]malononitrile
Properties
[(2-Chlorophenyl)methylene]malononitrile is a widely used riot control agent that belongs to the family of tear gases. This compound has a molecular formula of C10H5ClN2 and a molecular weight of 188.61 g/mol and is typically used in the form of a fine powder or aerosol. CS gas has a number of properties that make it an effective riot control agent, including its ability to cause severe irritation of the eyes, skin, and respiratory system in those who are exposed to it. It is also highly persistent and can remain in the environment for extended periods of time, making it difficult to remove once it has been deployed. However, the use of CS gas has also been associated with a number of health risks, particularly in individuals with pre-existing respiratory conditions such as asthma or chronic obstructive pulmonary disease (COPD). Exposure to this compound has been linked to a range of symptoms, including coughing, wheezing, and shortness of breath. In conclusion, while [(2-Chlorophenyl)methylene]malononitrile is highly effective as a riot control agent, its use carries significant health risks that must be carefully considered before deployment. 1
Applications
[(2-Chlorophenyl)methylene]malononitrile, also known as CS gas or chlorobenzylidenemalononitrile, is a chemical compound commonly used as a riot control agent and as the active ingredient in tear gas. It is a defining component of tear gas (CS gas) and is widely utilized by law enforcement agencies and military forces for crowd control purposes. CS gas is primarily used to disperse and incapacitate individuals during civil unrest or riot situations. When released into the air, it causes irritation and inflammation of the mucous membranes, leading to tearing, coughing, and temporary discomfort. Its strong irritant effects make it an effective tool for dispersing crowds and discouraging further protests or aggressive behavior. Research has shown that CS gas has a low lethality potential, making it a preferred choice over more lethal alternatives. Its use is regulated by international conventions and guidelines to ensure its responsible deployment and to prevent excessive harm. Overall, [(2-Chlorophenyl)methylene]malononitrile serves as a critical tool for maintaining public order and safety in challenging situations. Its effectiveness as a riot control agent has been extensively studied and documented. 2
Safety
The compound [(2-Chlorophenyl)methylene]malononitrile, also known as CS tear gas, exhibits various hazards and safety concerns. It is toxic when inhaled or in contact with the skin, causing irritation to the eyes and mucous membranes. Inhalation of CS aerosol can lead to immediate conjunctivitis, blepharospasm, and respiratory discomfort. Prolonged exposure to high concentrations may result in pulmonary edema and severe bronchospasm. Although the flash point data is unavailable, it is likely combustible. CS tear gas is considered a poison when ingested, intraperitoneally, or intravenously, and moderate toxicity has been observed through inhalation. It is classified as a human skin and eye irritant, causing intense irritation. Mutation data has been reported. However, there is limited evidence linking CS exposure to carcinogenicity. Studies on animals did not find a significant relationship between tumors and CS dose, but nonneoplastic lesions were observed primarily in the nasal passages. CS tear gas is primarily used as a riot control agent. 3
Reference
1. National Center for Biotechnology Information. o-Chlorobenzylidene malononitrile. PubChem Compound Summary. 2023.
2. United States Department of Justice. Chemical Agents, Riot Control Agents, and Herbicides. 2008.
3. [(2-Chlorophenyl)methylene]malononitrile. Europa, 2023. EC / List no.: 220-278-9.
- Related articles
- Related Qustion
- [(2-Chlorophenyl)methylene]malononitrile: Burn Injury of Exposure and its Mechanisms Apr 18, 2024
Exposure to [(2-Chlorophenyl)methylene]malononitrile can cause burn injuries through flame, contact, and chemical mechanisms, necessitating diverse medical treatments.
- Uses of CS Jan 12, 2022
CS, or o-chlorobenzylidene malononitrile, is the current major riot control agent (RCA) used by U.S. military forces. It was originally synthesized in 1928 by Corson and Stoughton, and the U.S. Army designated the compound ‘CS’ for the auth
o-Chlorobenzylidene malononitrile
2698-41-1You may like
o-Chlorobenzylidene malononitrile manufacturers
- [(2-Chlorophenyl)methylene]malononitrile
-
![2698-41-1 [(2-Chlorophenyl)methylene]malononitrile](/ProductImageEN1/2025-04/Small/9e7149cd-0767-4809-9316-b16318941f3c.gif)
- 2025-12-14
- CAS:2698-41-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 2-Chlorobenzylidenemalononitrile
-

- $1.00 / 1kg
- 2025-06-20
- CAS:2698-41-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
- 2-Chlorobenzylidenemalononitrile
-

- $1.00 / 1BOU/Drum
- 2020-11-27
- CAS:2698-41-1
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1kg