A reference standard, or reference material, is a substance prepared for use as the standard in an assay, identification or purity test. It has a quality appropriate to its use. For new drug substances reference standards intended for use in assays, the impurities should be adequately identified and/or controlled and purity should be measured by a quantitative procedure.
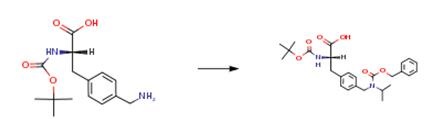
N-Boc-4-aminomethyl-L-phenylalanine - Reaction / Application on synthetic works
N-Boc-4-aminomethyl-L-phenylalanine is an important organic intermediate (building block) to synthetize substituted phenylalanine products.
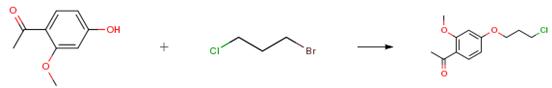
Nov 29,2019 Pharmaceutical Reference Standards1-(4-hydroxy-2-methoxyphenyl)ethanone- Reaction / Application on synthetic works
1-(4-hydroxy-2-methoxyphenyl)ethenone is an important organic building block to synthetize substituted methoxyphenyl products.
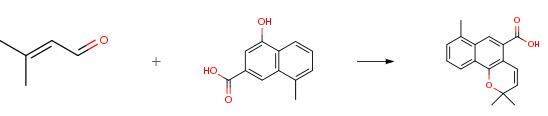
Nov 29,2019 Pharmaceutical Reference Standards8-methyl-4-hydroxy-2-naphthoic acid - Reaction / Application on Synthetic Works
8-Methyl-4-hydroxy-2-naphthoic acid is an important organic building block to synthetize substituted naphthoic products.
Nov 29,2019 Pharmaceutical Reference Standards