1-(4-hydroxy-2-methoxyphenyl)ethanone- Reaction / Application on synthetic works
Nov 29,2019
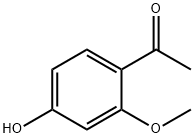
1-(4-hydroxy-2-methoxyphenyl)ethenone is an important organic building block to synthetize substituted methoxyphenyl products.
The following example is about its application on the synthesis of 1-[4-(3-Chloropropoxy)-2-methoxyphenyl]ethanone [1].
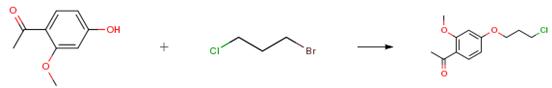
A mixture of 10.6 g (0.637 mole) of 1-(4-hydroxy-2-methoxyphenyl)ethanone, 20 g (0.127 mole) of 1-bromo-3-chloropropane and 26.4 g (0.19 mole) of anhydrous potassium carbonate in 250 ml of acetone was heated at reflux for 20 hr. The mixture was cooled, filtered and the filtrate concentrated under vacuum pump pressure at 90° C. to give an oil which gradually crystallized. The solid was triturated with petroleum ether (30°-60° C.), collected by filtration and dried to yield 14.6 g (94 percent) of white solid, m.p. 47°-49° C. on recrystallizing from isopropyl ether.
The following example is about its application on the synthesis of acyl hydrazone linkers [2].
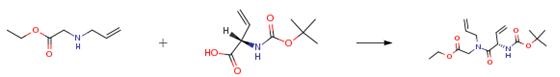
A 60 ml flask was charged with 4'-hydroxy-2'-methoxyacetophenone (1.2 g, 7.22 mmol) and potassium carbonate (1.497 g,10.83 mmol) then DMF (15 ml) was added. To this mixture, ethyl 4-bromobutyrate (1.240 ml,8.67 mmol) was added via a syringe. The mixture was heated at 100°C for 2h upon which LCMS showed completion. The mixture was cooled down then a large volume of water was added followed by EtOAc. The organic layer was washed with water then brine. It was dried over Na2SO4 then concentrated down. It was dried in the high vacuum to afford the intermediate ester product. This crude intermediate was dissolved in MeOH (15 ml) and treated with lithium hydroxide monohydrate (0.606 g,14.44 mmol) in 7.5 ml water and stirred at rt. After 2h, LCMS showed completion. The volatiles were evaporated. The residue was diluted with water then acidified with a solution of HCI (1 N) to low pH. A beige precipitate was formed. It was filtered and washed several times with water. It was dried under high vacuum to afford the product as a beige powder (1 .71 g, 94% yield).
The following example is about its application on the synthesis of novel lead compounds targeting fungal infections [3].
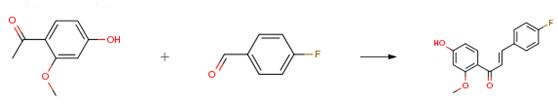
To a solution of the selected acetophenone (1.0 eq) and aldehyde (1.1 eq) in EtOH (10 mL)a KOH aqueous solution (50% p/v, 1 mL) was added dropwise and reaction mixture was stirred overnight at rt, diluted with H2O, and neutralized with aqueous 6N HCl. The formed solid/semisolid product was collected by vacuum filtration or extracted with a suitable solvent. The crude of reaction was then purified by column chromatography or by crystallization.
References
1.A. H. Robins Company, Incorporated. Arylalkylheterocyclic amines,N-substituted by aryloxyalkyl group in a method for allergy treatment. US4950674[P], 1990, A
2.Ontario Institute for Cancer Research (Oicr). Al-Awar R, Mamai A, Zhang A. Acyl hydrazone linkers, methods and uses thereof. WO2019/109188[P], 2019, A1, Paragraph 00235.
3.Bonvicini F, Gentilomi GA, Bressan F, Gobbi S, Rampa A, Bisi A, Belluti F. Functionalization of the chalcone scaffold for the discovery of novel lead compounds targeting fungal infections[J]. Molecules, 2019, 24(2): art. no. 372.
- Related articles
- Related Qustion
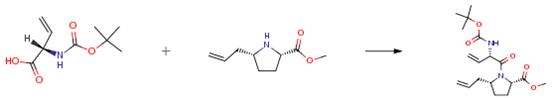
Boc-Vgl-OH is an important organic intermediate to synthetize substituted vinylglycine products.....
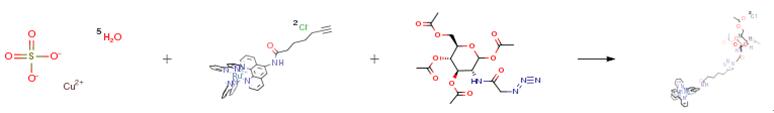
Nov 29,2019Amino Acids and Derivatives1,3,4,6-Tetra-O-acetyl-N-azidoacetylglucosamine is an important organic building block to synthetize substituted glucosamine products.....
Nov 29,2019Chemical ReagentsYou may like
1-(4-hydroxy-2-methoxyphenyl)ethanone manufacturers
- 1-(4-hydroxy-2-methoxyphenyl)ethanone
-

- $30.00 / 1KG
- 2025-12-12
- CAS:493-33-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20000KG
- 1-(4-hydroxy-2-methoxyphenyl)ethanone
-
- $0.00 / 1KG
- 2025-04-04
- CAS:493-33-4
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton
- 1-(4-hydroxy-2-methoxyphenyl)ethanone
-

- $15.00 / 1KG
- 2021-07-02
- CAS:493-33-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton