| Identification | More | [Name]
Ethoxymethylenemalononitrile | [CAS]
123-06-8 | [Synonyms]
2-(ETHOXYMETHYLENE)MALONONITRILE
2-(ETHOXYMETHYLIDENE)MALONONITRILE
ETHOXYMETHYLENEMALONITRILE
ETHOXYMETHYLENEMALONONITRILE
ETHOXYMETHYLENMALONONITRILE
LABOTEST-BB LT01148236
PROPANEDINITRILE, (ETHOXYMETHYLENE)-
(beta-Ethoxymethylene)malononitrile
(b-Ethoxymethylene)malononitrile
(ethoxymethylene)-malononitril
(ethoxymethylene)-propanedinitril
(Ethoxymethylene)propanedinitrile
1,1-Dicyano-2-ethoxyethene
1-Ethoxy-2,2-dicyanoethene
2-Cyano-3-ethoxyacrylonitrile
2-Ethoxy-1,1-ethenedicarbonitrile
a-Cyano-b-ethoxyacrylonitrile
alpha-Cyano-beta-ethoxyacrylonitrile
Ethoxymethylene-malonic acid dinitrile
Malononitrile, (ethoxymethylene)- | [EINECS(EC#)]
204-597-0 | [Molecular Formula]
C6H6N2O | [MDL Number]
MFCD00001854 | [Molecular Weight]
122.12 | [MOL File]
123-06-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Off White to Pale Yellow | [Melting point ]
64-66 °C(lit.)
| [Boiling point ]
160 °C12 mm Hg(lit.)
| [density ]
1.2236 (rough estimate) | [refractive index ]
1.4738 (estimate) | [Fp ]
155 °C
| [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
DMF, DMSO, Ethanol, Methanol | [form ]
Liquid | [color ]
Clear colorless to yellow | [Water Solubility ]
insoluble | [Detection Methods]
GC,NMR | [BRN ]
1634241 | [InChI]
1S/C6H6N2O/c1-2-9-5-6(3-7)4-8/h5H,2H2,1H3 | [InChIKey]
OEICGMPRFOJHKO-UHFFFAOYSA-N | [SMILES]
CCO\C=C(\C#N)C#N | [Uses]
Chemical intermediate. | [CAS DataBase Reference]
123-06-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Propanedinitrile, (ethoxymethylene)-(123-06-8) | [EPA Substance Registry System]
123-06-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,T,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R42/43:May cause sensitization by inhalation and skin contact .
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R43:May cause sensitization by skin contact.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S22:Do not breathe dust .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
3439 | [WGK Germany ]
3
| [RTECS ]
OO3850000
| [F ]
21 | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29269090 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral
Resp. Sens. 1
Skin Sens. 1 | [Toxicity]
mouse,LD50,intraperitoneal,50mg/kg (50mg/kg),National Technical Information Service. Vol. AD277-689, |
| Hazard Information | Back Directory | [Description]
Ethoxymethylenemalononitrile (EMMN) is an orange solid and the melting point of EMMN is 64–66 °C[1].It is an inexpensive reagent. As a functionalized malono-nitrile, it is widely used to synthesize pyrazoles, pyrimidines and a variety of fused heterocyclic systems. | [Chemical Properties]
Off White to Pale Yellow | [Preparation]
Triethyl orthoformate (67.3 mg, 0.454 mol) and malononitrile (20.0 mg,0.302 mol) were andded to acetic anhydride (77.2 gm, 0.75 moles) and the mixture was refluxed for 4-5 h at 110-140°C. The reaction was monitored by GC. After the reaction is complete, it is cooled to room temperature. Concentrated the mixture at 70°C at reduced pressure to yield crude solid product and the pure product (Ethoxymethylenemalononitrile) was obtained through purified either by vacuum distillation.
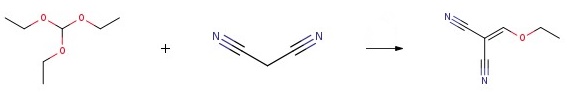 | [Synthesis Reference(s)]
Tetrahedron Letters, 10, p. 3279, 1969 DOI: 10.1017/S0009838800024678 | [Synthesis]
General procedure for the synthesis of 2-(ethoxymethylene)malononitrile from triethyl orthoformate and malononitrile: Triethyl orthoformate (67.3 g, 0.454 mol) was mixed with malononitrile (20.0 g, 0.302 mol) in acetic anhydride (77.2 g, 0.75 mol), and the reaction was carried out under reflux for 4-5 hours at 110-140 °C. The reaction process was monitored by gas chromatography (GC). Upon completion of the reaction, the reaction mixture was cooled to room temperature. Subsequently, the reaction mixture was concentrated under reduced pressure at 70 °C to afford the crude product 2-(ethoxymethylene)malononitrile. The crude product was further purified by vacuum distillation to give the pure 2-(ethoxymethylene)malononitrile. | [References]
[1] Faria J, et al. Ethoxymethylenemalononitrile. Synlett , 2013; 24: 0264–0265.
[2] Maurício , et al. Synthesis and antileishmanial evaluation of 1-aryl-4-(4,5-dihydro-1H-imidazol-2-yl)-1H-pyrazole derivatives. Bioorganic & Medicinal Chemistry Letters, 2011; 21: 7451-7454. |
|
|