| Identification | More | [Name]
SULFAMETHIZOLE | [CAS]
144-82-1 | [Synonyms]
4-AMINO-N-[5-METHYL-1,3,4-THIADIAZOL-2-YL]-BENZENESULFONAMIDE
LABOTEST-BB LT00772293
N1-(5-METHYL-1,3,4-THIADIAZOL-2-YL)SULFANILAMIDE
SULFAMETHIAZOLE
SULFAMETHIZOL
SULFAMETHIZOLE
SULFAMETHYLTHIADIAZOLE
SULPHAMETHIZOLE
2-(p-aminobenzenesulfonamido)-5-methylthiadiazole
2-methyl-5-sulfanilamido-1,3,4-thiadiazole
2-sulfanilamido-5-methyl-1,3,4-thiadiazole
4-amino-n-(5-methyl-1,3,4-thiadiazol-2-yl)-benzenesulfonamid
5-methyl-2-sulfanilamido-1,3,4-thiadiazole
ayerlucil
famet
lucosil
methazol
microsul
n(1)-(5-methyl-1,3,4-thiadiazol-2-yl)-sulfanilamid
n(sup1)-(5-methyl-1,3,4-thiadiazol-2-yl)-sulfanilamid | [EINECS(EC#)]
205-641-1 | [Molecular Formula]
C9H10N4O2S2 | [MDL Number]
MFCD00053363 | [Molecular Weight]
270.33 | [MOL File]
144-82-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
210 °C | [Boiling point ]
176°C (rough estimate) | [density ]
1.4704 (rough estimate) | [refractive index ]
1.6440 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Acetonitrile (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
neat | [pka]
5.45(at 25℃) | [color ]
White to Off-White | [Water Solubility ]
529mg/L(20 ºC) | [Merck ]
8916 | [BRN ]
255002 | [Major Application]
clinical testing | [InChI]
1S/C9H10N4O2S2/c1-6-11-12-9(16-6)13-17(14,15)8-4-2-7(10)3-5-8/h2-5H,10H2,1H3,(H,12,13) | [InChIKey]
VACCAVUAMIDAGB-UHFFFAOYSA-N | [SMILES]
Cc1nnc(NS(=O)(=O)c2ccc(N)cc2)s1 | [CAS DataBase Reference]
144-82-1(CAS DataBase Reference) | [EPA Substance Registry System]
Sulfamethizole (144-82-1) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R43:May cause sensitization by skin contact. | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves . | [WGK Germany ]
2
| [RTECS ]
WP0875000
| [TSCA ]
TSCA listed | [HS Code ]
2935904000 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Skin Sens. 1 | [Hazardous Substances Data]
144-82-1(Hazardous Substances Data) | [Toxicity]
LD50 oral in rat: 3500mg/kg |
| Hazard Information | Back Directory | [General Description]
White powder. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flammability data is lacking for this compound. SULFAMETHIZOLE is probably non-flammable. | [Description]
Sulfamethizole is a broad spectrum sulfonamide antibiotic (MIC90s = 1.25-5,000 μg/ml against clinical isolates of E. coli and K. pneumoniae).1 It inhibits dihydropteroate synthase, an enzyme involved in folate biosynthesis. Formulations containing sulfamethizole have previously been used to treat urinary tract infections. | [Originator]
Thiosulfil,Ayerst,US,1953 | [Uses]
Sulfamethizole is a sulfonamide based antibiotic that exhibit bactericidal activities towards gram-negative bacteria. Sulfamehizole was shown to be effective in treating gram-negative Bacillus AmpC en
zyme in elderly patients with lower respiratory tract infection and as well as against microbs responsible for tuberculosis. | [Uses]
Sulfonamide antibacterial. | [Uses]
This drug has antibacterial activity with respect to streptococci, pneumococci, staphylo�cocci, meningococci, gonococci, colon bacillus, pathogenic dysentery, and others. It is not
very toxic. It is generally used for acute, uncomplicated infections of the urinary tract that are caused by sensitive organisms. Because it is removed quickly from the organism by the
kidneys, the level of drug in the plasma remains low, and therefore it is not used for treat�ing infections that are localized in the urinary tract. Sulfisoxazole is the more preferred
drug. Synonyms of this drug are urosol, rufol, thiosulfil, and others. | [Definition]
ChEBI: A sulfonamide consisting of a 1,3,4-thiadiazole nucleus with a methyl substituent at C-5 and a 4-aminobenzenesulfonamido group at C-2. | [Manufacturing Process]
To 10 grams acetaldehyde-thiosemicarbazone in 80 grams pyridine gradually
20 grams p-acetaminobenzolsulfonyl chloride is added. The reaction mixture is
heated about 1 hour on a water bath and is then charged in 1 liter water, to
which some acetic acid is added. The bottom sediment is sucked off and
washed with water, after which it is crystallized by alcohol. 20 grams of the
condensation product thus obtained is cleared in 100 cc water at about 30°C,
after which 45 grams calcium ferricyanide dissolved in about 100 cc water is
added. The reaction mixture is made slightly alkaline and held at a
temperature of about 80°C for 2 to 3 hours. It is important that the reaction mixture during the whole period of 2 to 3 hours is steadily held alkaline.
After the said 2 to 3 hours the liquid is cooled and the bottom sediment,
which has a greenish color, is filtered off. The liquid sucked off eventually is
treated with active carbon, filtered and made slightly acid by means of acetic
acid, at which 2-amino-benzolsulfonamido-5-methyl-1,3,4-thiodiazol (melting
point 204° to 206°C) is precipitated. | [Brand name]
Thiosulfil (Wyeth);3p methazol;Amer-azo;Azocline;Azotrex;Dorsec;Lucatyl;Methisul;Micturol ampicilina seda;Nicene;Orozl;Procijec;Proklar-m;Rp 2145;S-methizole;Spasmo-harnosal;Starisil;Suladyne;Sulfa gram;Thiosulfil a;Tiosulfan;Uratrac;Urolex;Uroluxcosil;Uro-nebactin;Uropeutic;Urotrex. | [Therapeutic Function]
Antibacterial | [World Health Organization (WHO)]
Sulfamethizole, a sulfonamide anti-infective agent, was
introduced in 1953 for the treatment of bacterial infections. The importance of
sulfonamides has subsequently decreased as a result of increasing bacterial
resistance and their replacement by antibiotics which are generally more active
and less toxic. However sulfamethizole, which is rapidly eliminated, retains a place
in the treatment of urinary infections in some countries whereas in others its use has been discontinued. | [Pharmaceutical Applications]
2-Sulfanilamido-5-methyl-1,3,4-thiodiazole. A short-acting
sulfonamide (plasma half-life 2.5 h). Protein binding is c. 85%.
About 60% is excreted in the urine within 5 h. It was formerly
widely used in the treatment of urinary tract infection. | [Synthesis]
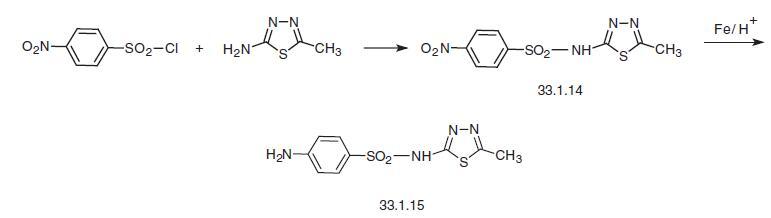
Sulfamethizole, N1-(5-methyl-1,3,4-thiadiazole-2-yl)sulfanilamide
(33.1.15), is synthesized in two ways. According to the first, 5-amino-2-methyl-1,3,4-
thiadiazole is reacted with 4-nitrobenzenesulfonyl chloride to make a nitro derivative
(33.1.14), which is then reduced using iron filings in acetic acid to give the desired sulfamethizole.
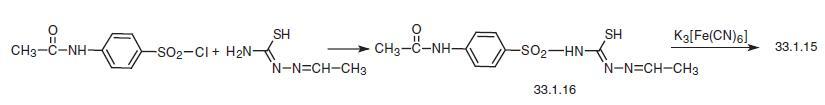
The second method of making sulfamethizole consists of reacting 4-acetylaminobenzene sulfonyl chloride with thiosemicarbazone of acetaldehyde, and subsequent oxidative
cyclization of the product (33.1.16) to the substituted 1,3,4-thiadiazole in the presence of
potassium ferricyanide in base, along with the simultaneous removal of the protective
acetyl group.
 | [References]
[1] Patent: WO2011/82400, 2011, A2. Location in patent: Page/Page column 52 |
|
|