| Identification | More | [Name]
2-Methoxypyridine | [CAS]
1628-89-3 | [Synonyms]
2-METHOXYPYRIDINE
METHYL 2-PYRIDYL ETHER
2-Methoxypyridine98%
2-METHOXY PYRIDINE FEMA NO.--------
2-Pyridol methyl ether | [EINECS(EC#)]
216-623-8 | [Molecular Formula]
C6H7NO | [MDL Number]
MFCD00006262 | [Molecular Weight]
109.13 | [MOL File]
1628-89-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Clear colorless to slightly yellow liquid | [Boiling point ]
142 °C (lit.) | [density ]
1.038 g/mL at 25 °C(lit.)
| [FEMA ]
4639 | 2-METHOXYPYRIDINE | [refractive index ]
n20/D 1.503(lit.)
| [Fp ]
90 °F
| [storage temp. ]
Flammables area | [form ]
Liquid | [pka]
3.28(at 20℃) | [color ]
Clear colorless to slightly yellow | [Odor]
at 0.10?%?in?propylene glycol. green fermented tea | [Water Solubility ]
INSOLUBLE | [Detection Methods]
GC,NMR | [JECFA Number]
2156 | [BRN ]
108189 | [InChI]
1S/C6H7NO/c1-8-6-4-2-3-5-7-6/h2-5H,1H3 | [InChIKey]
IWTFOFMTUOBLHG-UHFFFAOYSA-N | [SMILES]
COc1ccccn1 | [LogP]
1.32 | [CAS DataBase Reference]
1628-89-3(CAS DataBase Reference) | [NIST Chemistry Reference]
Pyridine, 2-methoxy-(1628-89-3) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,F | [Risk Statements ]
R10:Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 1993 3/PG 3
| [WGK Germany ]
3
| [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29339900 | [Storage Class]
3 - Flammable liquids | [Hazard Classifications]
Eye Irrit. 2
Flam. Liq. 3
Skin Irrit. 2
STOT SE 3 |
| Hazard Information | Back Directory | [Chemical Properties]
Clear colorless to slightly yellow liquid | [Uses]
2-Methoxypyridine can be used as an intermediate to synthesize 2-methoxy-3-pyridinesulfonyl chloride which is patented as a reactant used to prepare erythromycin macrolide antibiotics as antibacterial and antiprotozoal agents. It can also be used to prepare bicyclic iminopyrimidinones as Beta Amyloid Cleaving Enzyme-1 (BACE1) inhibitors. | [Synthesis]
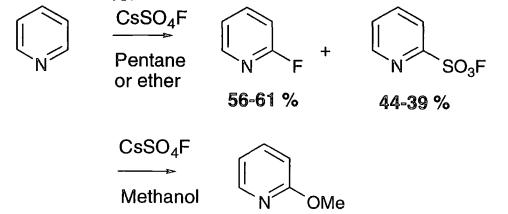
CsSO4F may be used to fluorinate pyridine directly, but the reaction is very sensitive to the solvents employed. When solvents such as pentane and diethyl ether were used, 2-fluoropyridine was the major product, but use of nucleophilic solvents, such as methanol gave 2-methoxypyridine.
 | [References]
[1] DAI W, LIU S, ZHANG Z, et al. Conformational preferences and isomerization upon excitation/ionization of 2-methoxypyridine and 2-N-methylaminopyridine†[J]. Physical Chemistry Chemical Physics, 2018, 9: 6211-6226. DOI:10.1039/C7CP07854D.
[2] Singh, Avtar, et al. "Synthesis of C-6 and C-3 substituted chalcogen derivatives of 2-methoxypyridine through lithiation of 2-methoxypyridine: An experimental and quantum chemical study." Inorganica Chimica Acta 432 (2015): 109-114.
[3] Trécourt, F., et al. "Catalyzed metalation applied to 2-methoxypyridine." The Journal of Organic Chemistry 53.7 (1988): 1367-1371.
[4] Ahipa, T. N., and Airody Vasudeva Adhikari. "2-Methoxypyridine derivatives: synthesis, liquid crystalline and photo-physical properties." New Journal of Chemistry 38.10 (2014): 5018-5029.
[5] WANYING CHENG . Conformation and bonding of 2-methoxypyridine and its monohydrate from rotational spectra[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2020, 239: Article 118434. DOI:10.1016/j.saa.2020.118434. |
|
|