| Identification | Back Directory | [Name]
Baloxavir marboxil | [CAS]
1985606-14-1 | [Synonyms]
CS-2794
Xofluza
S-033188
S-033188;XOFLUZA
Baloxavirmarboxi
aloxavir marboxil
2'S)-Folinic acid
Baoxavir
Marboxdil
Baloxavir marboxil
IBaloxavir marboxi
Baloxavir marboxil1
Baloxavir Impurity 9
PubChem ID: 124081896
Baloxavir marboxil API
Baloxavir Marboxil In-House
baloxavir marboxil S-033188
baloxavirmarboxil impurities
1985606-14-1 Baloxavir marboxil
Baloxavir marboxil (BXM, S033188)
N - [(s) - (2,3,4,5,6-pentafluorophenoxy) phenoxyphosphoryl] - L-alanine isopropyl ester (pentafluoroside chain)
(3R)-2-[(11S)-7,8-difluoro-6,11-dihydrobenzo[c][1]benzothiepin-11-yl]-9,12-dioxo-5-oxa-1,2,8-triazatricyclo[8.4.0.03,8]tetradeca-10,13-dien-11-yl]oxymethyl methyl carbonate
(((R)-12-((S)-7,8-Difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl)-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl)oxy)methyl methyl carbonate
Carbonic acid, [[(12aR)-12-[(11S)-7,8-difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl]-3,4,6,8,12,12a-hexahydro-6,8-dioxo-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl]oxy]methyl methyl ester
{[(3R)-2-[(2S)-12,13-difluoro-9-thiatricyclo[9.4.0.03,?]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-yl]-9,12-dioxo-5-oxa-1,2,8-triazatricyclo[8.4.0.03,?]tetradeca-10,13-dien-11-yl]oxy}methyl methyl carbonate | [EINECS(EC#)]
606-227-7 | [Molecular Formula]
C27H23F2N3O7S | [MDL Number]
MFCD31619272 | [MOL File]
1985606-14-1.mol | [Molecular Weight]
571.55 |
| Questions And Answer | Back Directory | [Description]
Baloxavir marboxil is an antiviral drug developed by Shionogi Co., a Japanese pharmaceutical company and Roche for the treatment of influenza A and influenza B infections. The drug was initially approved for use in Japan in February 2018 and approved by the FDA on October 24, 2018 for the treatment of acute uncomplicated influenza in patients 12 years of age and older who have been symptomatic for no more than 48 hours Label. Baloxavir marboxil, a cap-endonuclease inhibitor, has a unique mechanism of action when compared to the currently existing neuraminidase inhibitor drug class used to treat influenza infections.
| [Pharmacokinetics]
Baloxavir marboxil is a selective inhibitor of influenza cap-dependent endonuclease which prevents polymerase function and therefore influenza virus mRNA replication 5, 3. It has shown therapeutic activity against influenza A and B virus infections, including strains resistant to current antiviral agents 1. This drug inhibits an enzyme required for viral replication, thus rapidly treating flu virus infection 5, Label and alleviating the symptoms associated with infection. A single dose of this agent was shown to be superior to placebo in relieving influenza symptoms and superior to both oseltamivir and placebo drug in virologic outcomes (marked by decreased viral load).
| [Uses]
Baloxavir marboxil is an influenza medication, an antiviral,that is taken as a single dose tablet,by mouth, by individuals that are 12 years of age or older,that have presented symptoms of this infection for no more than 48 hours.The efficacy of baloxavir marboxil administered after 48 hours has not been tested.
| [Contraindications]
Baloxavir marboxil should not be co-administered with dairy products, calcium-fortified beverages, or laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminum or zinc. | [Metabolism]
Baloxavir marboxil is a prodrug that is converted by hydrolysis to baloxavir, the active form that exerts anti-influenza virus activity Label.
|
| Chemical Properties | Back Directory | [Boiling point ]
712.8±70.0 °C(Predicted) | [density ]
1.57±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Store in freezer, under -20°C | [solubility ]
DMSO:45.0(Max Conc. mg/mL);78.73(Max Conc. mM) | [form ]
A crystalline solid | [pka]
-1.46±0.40(Predicted) | [color ]
White to yellow | [InChIKey]
HKVHAHZGMLTCDW-BWFGELNCSA-N | [SMILES]
C(O)(=O)OC(OC1=C2N(C=CC1=O)N([C@H]1C3=CC=C(F)C(F)=C3CSC3=CC=CC=C31)[C@]1([H])COCCN1C2=O)C |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Zofluza intermediate-->3,4-Difluorobenzoic acid-->7,8-difluorodibenzo[B,E]thiazepine-11(6H)-one-->2-(2-aminoethoxy)-1,1-dimethoxyethane-->Methanesulfonic acid-->(R)-(+)-Tetrahydro-2-furoic acid-->(R)-7-(benzyloxy)- 3,4,12,12a-tetrahydro- 1H-[1,4]oxazino[3,4- c]pyrido[2,1-f][1,2,4]- triazine-6,8-dione-->allyl 3-methoxymorpholine-4-carboxylate-->3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid-->1-Amino-3-benzyloxy-4-oxo-1,4-dihydropyridine-2-carboxylic acid ethyl ester-->Allyl 3-((3-(benzyloxy)-2-(ethoxycarbonyl)-4-oxopyridin-1(4H)-yl)amino)morpholine-4-carboxylate | [Preparation Products]
Baloxavir |
| Hazard Information | Back Directory | [Preparation]
The synthesis of baloxavir marboxil involved the following steps: The coupling of the 1-R and 2 fragments was carried out under dehydration conditions of 1-propanephosphonic anhydride (T3P) and methanesulfonic acid at 70 °C to obtain protected baloxavir 19. Compound 19 was then reacted with 0.6 equivalents of PhBr and K2CO3. Debenzylation was then carried out using LiCl in CH3CONMe2 to give baloxavir acid in 94% yield. In the final step for the preparation of the prodrug, baloxavir acid was reacted with chloromethyl methyl carbonate in dimethylacetamide in 93% yield to form baloxavir marboxyl.
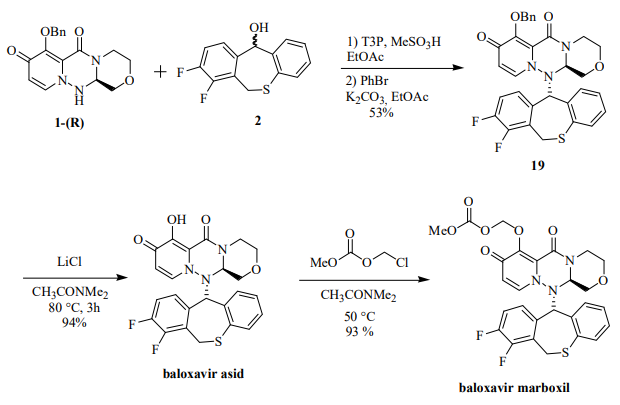
The reaction mechanism for the final step for the synthesis of baloxavir marboxil(BXM). | [Brand name]
XOFLUZA? (baloxavir marboxil) | [Biological Activity]
Baloxavir marboxil is a prodrug that is metabolised to the active form baloxavir acid also known as S-033447. S-033447 is a small molecule inhibitor of the cap-dependent endonuclease of influenza A and B viruses. It has shown nanomolar antiviral activity against influenza A and B viruses in vitro. In murine models of seasonal influenza and avian influenza A(H5N1) or A(H7N9), orally administered baloxavir showed a rapid reduction in pulmonary viral loads and decreased mortality. Baloxavir significantly reduced the time for alleviation of symptoms and reduced virus titres at 24 and 48 hours post-treatment at three different doses (10 mg, 20 mg and 40 mg) in a phase II study with patients experiencing uncomplicated influenza. | [Mechanism of action]
Baloxavir marboxil is an influenza therapeutic agent, specifically, an enzyme inhibitor targeting the influenza virus' cap-dependent endonuclease activity, one of the activities of the virus polymerase complex. In particular, it inhibits a process known as cap snatching, by which the virus derives short, capped primers from host cell RNA transcripts, which it then uses for polymerase-catalyzed synthesis of its needed viral mRNAs. A polymerase subunit binds to the host pre-mRNAs at their 5' caps, then the polymerase's endonuclease activity catalyzes its cleavage "after 10–13 nucleotides". As such, its mechanism is distinct from neuraminidase inhibitors such as oseltamivir and zanamivir. | [Side effects]
Common side effects following the single dose administration of baloxavir marboxil include diarrhea, bronchitis, common cold, headache, and nausea. Adverse events were reported in 21% of people who received baloxavir, 25% of those receiving placebo, and 25% of oseltamivir. | [References]
[1] HUGHES* D L. Review of the Patent Literature: Synthesis and Final Forms of Antiviral Drugs Tecovirimat and Baloxavir Marboxil[J]. Organic Process Research & Development, 2019, 23 7: 1298-1307. DOI:10.1021/acs.oprd.9b00144.
[2] ANDOYOSHINORI. Pharmacokinetic and pharmacodynamic analysis of baloxavir marboxil, a novel cap-dependent endonuclease inhibitor, in a murine model of influenza virus infection.[J]. Journal of Antimicrobial Chemotherapy, 2021, 76 1: 189-198. DOI:10.1093/jac/dkaa393.
[3] YANGTIANRUI. Baloxavir Marboxil: The First Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza.[J]. Annals of Pharmacotherapy, 2019, 53 7: 754-759. DOI:10.1177/1060028019826565.
[4] Chemical Structure and Synthesis of Baloxavir Marboxil, A Novel Endonuclease Inhibitor For The Treatment Influenza : An Overview |
|
|