| Identification | Back Directory | [Name]
CPD2206 | [CAS]
2152628-33-4 | [Synonyms]
CPD2206
LOX-292
LOXO-292
SELPERCATINIB
LOXO-292 USP/EP/BP
Inhibitor LOXO-292
LOXO-292,Selpercatinib
Selpercatinib(LOXO-292)
LOXO-292; LOXO292;LOXO 292
6-(2-hydroxy-2-methylpropoxy)-4-[6-[6-[(6-methoxypyridin-3-yl)methyl]-3,6-diazabicyclo[3.1.1]heptan-3-yl]pyridin-3-yl]pyrazolo[1,5-a]pyridine-3-carbonitrile
Pyrazolo[1,5-a]pyridine-3-carbonitrile, 6-(2-hydroxy-2-methylpropoxy)-4-[6-[6-[(6-methoxy-3-pyridinyl)methyl]-3,6-diazabicyclo[3.1.1]hept-3-yl]-3-pyridinyl]-
6-?(2-?Hydroxy-?2-?methylpropoxy)?-?4-?[6-?[6-?[(6-?methoxy-?3-?pyridinyl)?methyl]?-?3,?6-?diazabicyclo[3.1.1]?hept-?3-?yl]?-?3-?pyridinyl]?pyrazolo[1,?5-?a]?pyridine-?3-?carbonitrile | [EINECS(EC#)]
207-854-9 | [Molecular Formula]
C29H31N7O3 | [MDL Number]
MFCD31814089 | [MOL File]
2152628-33-4.mol | [Molecular Weight]
525.6 |
| Chemical Properties | Back Directory | [density ]
1.36±0.1 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO:20.0(Max Conc. mg/mL);38.05(Max Conc. mM) | [form ]
A crystalline solid | [pka]
14.00±0.29(Predicted) | [color ]
White to off-white | [InChIKey]
XIIOFHFUYBLOLW-UHFFFAOYSA-N | [SMILES]
C12=C(C#N)C=NN1C=C(OCC(O)(C)C)C=C2C1=CC=C(N2CC3CC(N3CC3=CC=C(OC)N=C3)C2)N=C1 |
| Hazard Information | Back Directory | [Uses]
Selpercatinib is a small molecule protein kinase inhibitor which may be used in the treatment of cancer. | [Brand name]
Retevmo | [General Description]
Class: receptor tyrosine kinase;
Treatment: RET-altered lung/thyroid cancers;
Other name: ARRY-192, LOXO-292;
Oral bioavailability = 73%;
Elimination half-life = 32 h;
Protein binding = 97% | [Side effects]
Selpercatinib can cause serious side effects including liver toxicity, high blood pressure, heart rhythm changes due to prolongation of heart electrical activity (QT prolongation), bleeding, allergic reactions, impaired wound healing and harm to an unborn baby.
Selpercatinib may cause harm to a developing fetus or a newborn baby. | [Synthesis]
The sulfonyl chloride starting material 329 was reacted with tert-butyl hydroxyurea (330) under basic conditions and subsequently treated with trifluoroacetic acid (TFA) to afford O-sulfonylhydroxylamine 332. O-sulfonylhydroxylamine 332, as a nitrogen source, reacted with pyridine 333 to afford N-aminopyridinium salt 334. N-aminopyridinium salt 334 was further reacted with 2-chloroacrylonitrile (335) to establish the pyrazolopyridine core 336. Removal of the methyl ether using dodecanethiol (337) in warm dimethylformamide under basic conditions afforded the phenolic compound 338. Phenol 338 was converted to the triflate (339). The triflate 339 was cross-coupled with the boronate ester 340 under Suzuki conditions to react specifically at the triflate position with retention of the bromide to afford 341. 341 was reacted with the epoxide 342 under palladium catalysis to establish the ether side chain. Finally, the azabicyclic addendum 344 was installed via an NMR aromatic substitution reaction to generate salacartinib in 86% yield.

| [target]
RET | [storage]
Store at -20°C | [Dosage]
The recommended selpercatinib dose based on body weight is:
(1) Less than 50 kg: 120 mg orally twice daily
(2) 50 kg or greater: 160 mg orally twice daily
|
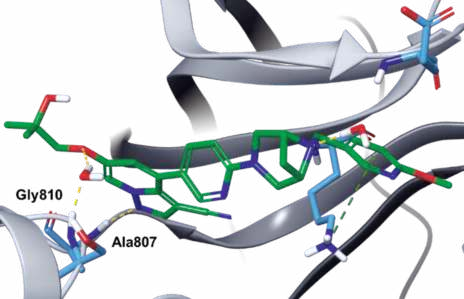
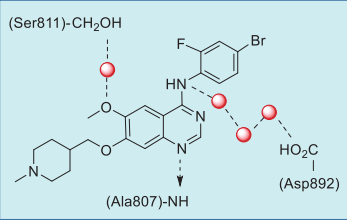
| Questions And Answer | Back Directory | [Binding Mode]
In the co-crystal structure of selpercatinib-RET
kinase, the pyrazolopyridine occupies the
adenosine pocket, forming a direct hydrogen bond
between N1 and amide NH of Ala807 and an indirect
hydrogen bond between C6 ether oxygen and amide
NH of Gly810 via a bound water molecule. In addition,
one piperazine nitrogen also interact with one water
molecule (Figs. 3, 4).
  |
|
|