| Identification | Back Directory | [Name]
3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]-2-Pyridinamine | [CAS]
877399-52-5 | [Synonyms]
Xalkori
Crizotinib
PF 2341066
Crozotinib
PF-02341066
PF-2341066/Crizotinib
Crizotinib (PF2341066)
PF-02341066 Crizotinib
Crizotinib,PF-02341066
k-ras(g12c) inhibitor 6 NA
Crizotinib
3-[1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine
3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]-2-Pyridinamine
(R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine
3-[(1R)-1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]pyridin-2-amine
3-((R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine
(R)-3-[1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)pyridin-2-ylaMine
3-[(1R)-1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]-2-aMinopyridine
2-PyridinaMine, 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]-
[3-[[(R)-1-(2,6-Dichloro-3-
fluorophenyl)ethyl]oxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-yl]aMine | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C21H22Cl2FN5O | [MDL Number]
MFCD12407409 | [MOL File]
877399-52-5.mol | [Molecular Weight]
450.343 |
| Chemical Properties | Back Directory | [Melting point ]
192 °C | [Boiling point ]
599.2±50.0 °C(Predicted) | [density ]
1.47±0.1 g/cm3(Predicted) | [storage temp. ]
room temp | [solubility ]
Soluble in DMSO (up to 25 mg/ml with warming) or in Ethanol (up to 25 mg/ml with warming) | [form ]
powder | [pka]
9.81±0.10(Predicted) | [color ]
white to tan | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. | [InChIKey]
KTEIFNKAUNYNJU-GFCCVEGCSA-N | [SMILES]
C1(N)=NC=C(C2=CN(C3CCNCC3)N=C2)C=C1O[C@@H](C1=C(Cl)C=CC(F)=C1Cl)C | [CAS DataBase Reference]
877399-52-5 |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Usage]
A potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). A potential antitumor agent. | [Usage]
Crizotinib is a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). Crizotinib is a potential antitumor agent. | [Usage]
PF-2341066 (Crizotinib) is a potent inhibitor of c-Met and ALK with IC50 of 11 nM and 24 nM, respectivley | [Definition]
ChEBI: A 3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)pyrazol-4-yl]pyridin-2-amine that has R configuration at the chiral centre. The active enantiomer, it acts as a kinase inhibitor and is used for the treatment of patients wi
h locally advanced or metastatic non-small cell lung cancer (NSCLC) | [Description]
In August 2011, the United States FDA approved crizotinib (PF-
02341066) for the treatment of anaplastic lymphoma kinase (ALK)
rearranged non-small-cell lung cancer (NSCLC).
Crizotinib is a dual ATP competitive inhibitor of tyrosine kinases c-MET (Mesenchymal-Epithelial Transition Factor)
kinase (cellular IC50=8 nM) and ALK (cellular IC50=20 nM), both of
which are important targets for cancer chemotherapy. When crizotinib
was tested for selectivity versus other kinases it was found to have enzyme
IC50's within 100-fold multiples of c-MET for 13 of the 120 kinases tested.
In cellular assays, crizotinib was found to inhibit RON (recepteur d’origine
nantais) kinase with a 10-fold selectivity window over c-MET. | [Originator]
Pfizer (United States) | [Uses]
A potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). A potential antitumor agent. | [Uses]
Crizotinib is a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). Crizotinib is a potential antitumor agent. | [Uses]
PF-2341066 (Crizotinib) is a potent inhibitor of c-Met and ALK with IC50 of 11 nM and 24 nM, respectivley | [Indications]
Crizotinib (Xalkori(R), Pfizer), approved in 2011, was the first approved inhibitor targeting anaplastic lymphoma kinase (ALK). ROS protooncogene 1-encoded kinase (ROS1) of the tyrosine kinase insulin receptor class and MET proto-oncogene-encoded kinase of the hepatocyte growth factor receptor (HGFR) class are other kinases targeted by crizotinib.When approved in 2011, crizotinib was the first drug specifically targeting NSCLC patients. However, resistance to crizotinib was usually observed in approximately 8 months after initial application and more than half of crizotinib-treated patients experienced gastrointestinal side effects. In 2016,crizotinib was additionally approved for ROS1-positive NSCLC by FDA. | [Brand name]
Xalkori | [General Description]
Class: receptor tyrosine kinase; Treatment: NSCLC; Oral bioavailability = 43%; Elimination half-life = 42 h; Protein binding = 90%
| [Biochem/physiol Actions]
Crizotinib (PF-02341066) is an ATP-competitive inhibitor of the receptor tyrosine kinases (RTKs) c-Met (hepatocyte growth factor receptor) and anaplastic lymphoma kinase (ALK). Crizotinib is a highly specific inhibitor of c-Met and ALK among > 120 different RTKs surveyed. Crizotinib was approved for treatment of a subtype of nonsmall-cell lung cancer (NSCLC) with ALK fusion mutations. | [Clinical Use]
More recent studies have shown that patients with MET amplification and no ALK rearrangement treated with crizotinib have responded well in NSCLC and squamous cell lung carcinoma.
Crizotinib is a potent and selective mesenchymal epithelial
transition factor/anaplastic lymphoma kinase (cMET/ALK) inhibitor. Marketed under the brand name Xalkori, crizotinib was discovered and developed by Pfizer and is approved for the treatment
of advanced or metastatic non-small cell lung cancer (NSCLC)
that is caused by the echinoderm microtubule associated proteinlike
4 (EML4) mutation of ALK. Crizotinib is also undergoing
clinical evaluation against additional cancers which express the
ALK mutation, such as advanced disseminated anaplastic large-cell
lymphoma and neuroblastoma. | [Side effects]
crizotinib (Xalkori) is an oral receptor tyrosine kinase inhibitor indicated for the treatment of patients with advanced or metastatic non-small cell lung cancer (NSCLC). Common side effects with Xalkori use include upper respiratory infection, nausea, vomiting, stomach pain, decreased appetite, insomnia, dizziness, tired feeling, diarrhea, constipation, rash or itching, cold symptoms (stuffy nose, sneezing, sore throat), numbness or tingling, or swelling in your hands or feet.
http://www.rxlist.com/xalkori-side-effects-drug-center.htm | [Synthesis]
Several synthetic routes for the
preparation of crizotinib have been reported, each employing a
very similar convergent strategy. The synthesis utilized to prepare
over 100 kg is described in the scheme.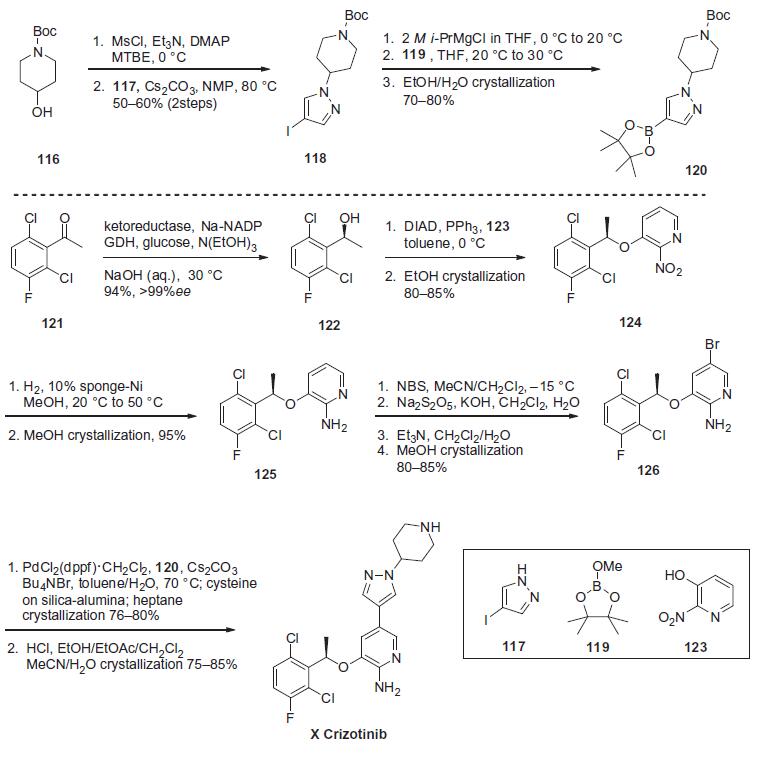
Mesylation of tert-butyl-4-hydroxypiperidine-1-carboxylate
(116) followed by displacement with 4-iodopyarazole (117) provided
iodopyrazine 118 in 50¨C60% overall yield for the two steps.
Reaction of iodide 118 with i-PrMgCl furnished the corresponding
Grignard reagent, which was quenched with borolane 119 to give
the arylboronate 120 in 70¨C80% yield after crystallization from
ethanol/water. The Suzuki coupling partner of 120 (bromide 126)
was prepared in several steps starting with enzymatic reduction of 2,6-dichloro-3-fluoroacetophenone (121) using an engineered
ketoreductase process, providing alcohol 122 in 94% yield and in
>99% ee. Mitsunobu reaction with 3-hydroxy-2-nitropyridine
(123) provided nitropyridine 124 in 80¨C85% yield after crystallization
from ethanol and with no loss in enantiopurity. Chemoselective
reduction of the nitro group was accomplished through
hydrogenation using 10% sponge-nickel catalyst to give amine
125 in 95% yield after crystallization from methanol. Regioselective
bromination of 125 using NBS in CH3CN/CH2Cl2, followed by a
bisulfate quench and Et3N wash (to purge residual succinimide)
and subsequent crystallization from methanol provided Suzuki-
Miyaura coupling partner 126 in 80¨C85% yield. Coupling of arylbromide
126 with arylboronate 120 was accomplished using
0.8 mol % PdCl2(dppf)�CH2Cl2 as the catalyst, followed by treatment
with cysteine on silica-alumina to purge residual palladium. Crystallization
of the resulting mixture from heptanes provided the
coupled product in 76¨C80% yield, which upon acid-promoted removal of the Boc protecting group and crystallization from
CH3CN/H2O produced crizotinib (X) in 75¨C80% yield. | [target]
Primary targets: ALK/ROS1/MET | [Drug interactions]
Potentially hazardous interactions with other drugs
Analgesics: use alfentanil and fentanyl with caution. Antibacterials: concentration reduced by rifabutin
and rifampicin - avoid; concentration increased by
clarithromycin and telithromycin - avoid.
Antidepressants: St John’s wort may reduce
concentration of crizotinib - avoid.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin, phenobarbital and
phenytoin - avoid.
Antifungals: concentration increased by ketoconazole
and possibly with itraconazole and voriconazole -
avoid.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis); avoid with pimozide.
Antivirals: concentration possibly increased by
atazanavir, indinavir, ritonavir and saquinavir -
avoid.
Anxiolytics and hypnotics: increases concentration of
midazolam.
Ciclosporin: use with caution.
Cytotoxics: possibly increases ibrutinib concentration
- reduce dose of ibrutinib.
Ergot alkaloids: use with caution.
Grapefruit juice: may increase concentration of
crizotinib, avoid.
Oestrogens and progestogens: contraceptive effect
possibly reduced - avoid.
Sirolimus: use with caution.
Tacrolimus: use with caution. | [Metabolism]
Mainly metabolised in the liver by CYP3A4/5. The main
metabolic pathways are oxidation (to crizotinib lactam)
and O-dealkylation.
Excreted 53% via faeces (53% unchanged) and 22% via
urine (2% unchanged). | [storage]
Store at -20°C | [Mode of action]
Crizotinib is an inhibitor of receptor tyrosine kinases including ALK, Hepatocyte Growth Factor Receptor (HGFR, c-Met), and Recepteur d'Origine Nantais (RON). Translocations can affect the ALK gene resulting in the expression of oncogenic fusion proteins. The formation of ALK fusion proteins results in the activation and dysregulation of the gene's expression and signaling, which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib demonstrates concentration-dependent inhibition of ALK and c-Met phosphorylation in cell-based assays using tumor cell lines, and also demonstrates antitumor activity in mice bearing tumor xenografts that express EML4-or NPM-ALK fusion proteins or c-Met.Crizotinib is a multitargeted small molecule tyrosine kinase inhibitor, which had been originally developed as an inhibitor of the mesenchymal epithelial transition growth factor (c-MET); it is also a potent inhibitor of ALK phosphorylation and signal transduction. This inhibition is associated with G1-S phase cell cycle arrest and induction of apoptosis in positive cells in vitro and in vivo. Crizotinib also inhibits the related ROS1 receptor tyrosine kinase.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3876666/ | [References]
1) Christensen et al. (2007), Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma; Mol. Cancer Ther., 6 3314
2) Zou et al. (2007), An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms; Cancer Res., 67 4408
3) Bang et al. (2010), Clinical activity of the oral ALK inhibitor PF-02341066 in ALK-positive patients with non-small cell lung cancer (NSCLC); J. Clin. Oncol., 28 3 |
|
|