| Identification | Back Directory | [Name]
NITROCYCLOPENTANE | [CAS]
2562-38-1 | [Synonyms]
NITROCYCLOPENTANE
nitro-cyclopentan
1-Nitrocyclopentane
NITROCYCLOPENTANE, 97+%
(1S,2S)-Methyl 2-aMinocyclopropanecarboxylate | [EINECS(EC#)]
219-884-6 | [Molecular Formula]
C5H9NO2 | [MDL Number]
MFCD00001362 | [MOL File]
2562-38-1.mol | [Molecular Weight]
115.13 |
| Chemical Properties | Back Directory | [Appearance]
Clear pale yellow liquid | [Melting point ]
143-144 °C | [Boiling point ]
180 °C(lit.)
| [density ]
1.086 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.454(lit.)
| [Fp ]
153 °F
| [form ]
liquid | [pka]
8.46±0.20(Predicted) | [BRN ]
907079 | [InChI]
1S/C5H9NO2/c7-6(8)5-3-1-2-4-5/h5H,1-4H2 | [InChIKey]
CJSZWOGCKKDSJG-UHFFFAOYSA-N | [SMILES]
[O-][N+](=O)C1CCCC1 | [CAS DataBase Reference]
2562-38-1 |
| Hazard Information | Back Directory | [Chemical Properties]
Clear pale yellow liquid | [Uses]
Nitrocyclopentane, a secondary nitroalkane, was examined for its ability to induce DNA repair in rat hepatocytes. The nitronate of nitrocyclopentane was mutagenic in Salmonella strains TA100 and TA102. | [Preparation]
Bromocyclopentane (22.0 g, 0.15 mol) was added to a soln of NaNO2 (18 g, 0.26 mol) in dry DMSO (100 mL) at 15 ℃ and the mixture was stirred at this temperature for 3 h. The mixture was poured into ice water (250 mL) and extracted with petroleum ether (bp 35-37 ℃; 4 × 50 mL). The combined organic extracts were washed with H2O (4× 50 mL), dried (MgSO4), and concentrated under reduced pressure. The residue was distilled to give Nitrocyclopentane; yield: 9.9 g (58%); bp 62 ℃/8 Torr; nD/20 1.4538.
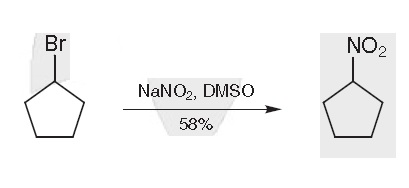
Synthesis of Nitrocyclopentane | [Synthesis Reference(s)]
Tetrahedron Letters, 21, p. 1117, 1980 DOI: 10.1016/S0040-4039(01)83928-9 | [General Description]
Nitrocyclopentane, a secondary nitroalkane, was examined for its ability to induce DNA repair in rat hepatocytes. The nitronate of nitrocyclopentane was mutagenic in Salmonella strains TA100 and TA102. |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
36/37/38 | [Safety Statements ]
26-36/37/39 | [RIDADR ]
2810 | [WGK Germany ]
3
| [RTECS ]
GY4657500
| [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29042000 | [Storage Class]
10 - Combustible liquids | [Hazard Classifications]
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| Questions And Answer | Back Directory | [Application]
Nitroalkanes are primarily used as raw materials for organic synthesis, solvents, and explosives, and can produce nearly 2,000 derivatives. Their applications span various sectors of the national economy, including pharmaceuticals, pesticides, explosives, dyes, reagents, solvents, surfactants, extractants, emulsifiers, and lubricants. The condensation of nitroalkanes with aldehydes, followed by reduction, yields many versatile amino alcohols, representing one of the most valuable industrial applications of nitroalkanes. |
|
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|