| Identification | Back Directory | [Name]
(R)-(+)-1-(4-PYRIDYL)ETHANOL | [CAS]
27854-88-2 | [Synonyms]
(1R)-1-(4-Pyridyl)ethanol
(R)-1-(pyridin-4-yl)ethanol
(R)-(+)-1-(4-PYRIDYL)ETHANOL
4-[(R)-1-Hydroxyethyl]pyridine
(R)-1-(pyridin-4-yl)ethan-1-ol
(1R)-1-(pyridin-4-yl)ethan-1-ol
(R)-4-(1-HYDROXYETHYL) PYRIDINE
(R)-α-Methylpyridine-4-methanol
(αR)-α-Methylpyridine-4-methanol
(aR)-a-Methyl-4-PyridineMethanol
4-PyridineMethanol, α-Methyl-, (R)-
(r)-(+)-α-methyl-4-pyridinemethanol
(R)-(+)-ALPHA-METHYL-4-PYRIDINEMETHANOL
(αR)-α-Methyl-4-pyridinemethanol,99%e.e.
(R)-4-(1-Hydroxyethyl)pyridine, (99+% ee)
(R)-(+)-alpha-Methyl-4-pyridinemethanol 99%
(R)-(+)-α-Methyl-4-pyridinemethanol, min. 98%
(R)-4-(1-Hydroxyethyl)pyridine,99+%,(99+% ee)
(R)-4-(1-Hydroxyethyl)pyridine, (99+% ee), 99+%
(R)-4-(1-Hydroxyethyl)pyridine(R)-(+)-1-(4-Pyridyl)ethanol
(R)-(+)-ALPHA-METHYL-4-PYRIDINEMETHANOL, 99% (98% EE/GLC) | [Molecular Formula]
C7H9NO | [MDL Number]
MFCD00077865 | [MOL File]
27854-88-2.mol | [Molecular Weight]
123.15 |
| Chemical Properties | Back Directory | [Appearance]
White needles | [Melting point ]
67-69 °C(lit.)
| [Boiling point ]
239.7±15.0 °C(Predicted) | [density ]
1.082±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
soluble in Methanol | [form ]
Crystalline Powder or Needles | [pka]
13.52±0.20(Predicted) | [color ]
White | [Optical Rotation]
[α]20/D +55°, c = 1 in chloroform | [BRN ]
4306037 | [InChI]
1S/C7H9NO/c1-6(9)7-2-4-8-5-3-7/h2-6,9H,1H3/t6-/m1/s1 | [InChIKey]
HVOAMIOKNARIMR-ZCFIWIBFSA-N | [SMILES]
C[C@@H](O)c1ccncc1 |
| Hazard Information | Back Directory | [Chemical Properties]
White needles | [Uses]
(1R)-1-(4-Pyridyl)ethanol is a building block reagent used in the synthesis of potent GPR119 receptor agonists used in the treatment of diabetes. | [Synthesis]
GENERAL PROCEDURE: BH3-SMe2 (2.0 M toluene solution, 250 μL, 500 μmol) was added to a pre-prepared toluene (1 mL) solution of B-alkoxyoxazaborolidine 8 (7.43 mg, 25.0 μmol) (refer to Procedure 1.2.6 for preparation). The reaction mixture was kept at 50°C for 15 min and then cooled to 20°C. Subsequently, a solution of acetophenone (1a, 60.1 mg, 58.3 μL, 500 μmol) in toluene (1 mL) was slowly added dropwise over 60 min using a syringe pump. After the dropwise addition, the reaction was continued with stirring for 10 min, and then the reaction was quenched by the addition of H2SO4 (1.1 M, 500 μL). The mixture was stirred for another 15 min and then directly separated by column chromatography (silica gel, pentane/Et2O 2:1) to afford the target product (R)-1-(pyridin-4-yl)ethanol ((R)-2a, 58.3 mg, 477 μmol, 95% yield, 97% ee) as a colorless oil. | [Purification Methods]
Purify it by recrystallisation from pet ether. The m recorded after sublimation was 59.9-60.2o, and 55o after crystallisation from *C6H6/pet ether or pet ether/*C6H6. The (-)-di-O-benzoyl tartrate salt has m 146-148o (from EtOH). [UV, ORD: Harelli & Samori J Chem Soc Perkin Trans 2 1462 1974.] The racemate recrystallises from Et2O with m 74-76o,b 90-94o/1mm. The picrate has m 125-126o (from *C6H6). [Ferles & Attia Collect Czech Chem Commun 38 611 1973, UV, NMR: Nielson et al. J Org Chem 29 2898 1964, Beilstein 21 III/IV 522, 21/2 V 217.] | [References]
[1] Organic Letters, 2017, vol. 19, # 3, p. 690 - 693
[2] Advanced Synthesis and Catalysis, 2014, vol. 356, # 10, p. 2293 - 2302
[3] Tetrahedron Letters, 2016, vol. 57, # 23, p. 2492 - 2495
[4] Synlett, 1997, vol. 1997, # 3, p. 273 - 274
[5] Synthesis, 2009, # 14, p. 2413 - 2417 |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
36/37/38 | [Safety Statements ]
26-36 | [WGK Germany ]
3
| [F ]
3-10 | [HS Code ]
29333999 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| Questions And Answer | Back Directory | [Reaction]
- Used for the synthesis of chiral 1-(4-pyridinyl)ethylamines
- Precursor for intermediates of highly stable chiral (A)6-B Supramolecular Copolymers
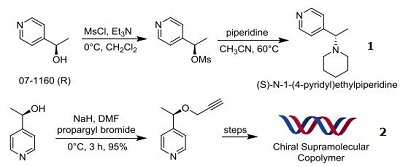
|
|
|