| Identification | More | [Name]
Teniposide | [CAS]
29767-20-2 | [Synonyms]
TENIPOSIDE
3’,4’:6,7]naphtho[2,3-d]-1,3-dioxol-6(5ah)-one
4’-demethyl1-o-(4,6-o,o-(2-thenylidene)-beta-d-glucopyranosyl)epipodophyllot
4’-demethyl1-o-(4,6-o,o-(2-thenylidene)-beta-d-glucopyranosyl)epipodophylloto
4’-demethylepipodophyllotoxin9-(4,6-o-2-thenylidene-bata-d-glucopyranoside.
4’-demethylepipodophyllotoxin9-(4,6-o-2-thenylidene-beta-d-glucopyranoside)
4’-demethyl-epipodophyllotoxin-beta-d-thenylidene-glucosid
4’-demethyl-epipodophyllotoxin-beta-d-thenylidene-glucoside
4’-demethylepipodophyllotoxin-beta-d-thenylidineglucoside
5abeta,8aalpha,9beta(r*)]]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-[5r-[5alph
dimethoxyphenyl)-9]]4-6-o-(2-thienylmethylene)-beta-d-glucopyranosyl]oxy]furo[
epipodophyllotoxin,4’-demethyl-,9-(4,6-o-2-thenylidene-beta-d-glucopyranoside
etp
nsc122819
nsc-122819
vehem
vm-26
Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-[[4,6-O-[(R)-2-thienylmethylene]-b-D-glucopyranosyl]oxy]-, (5R,5aR,8aR,9S)-
4'-Dimethylepipodophyllotoxin-9-(4,6-O-2-thenylidene)-b-D-glucopyranoside
[5R-[5alpha,5abeta,8aalpha,9beta(R*)]]-5,8,8a,9-Tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-[[4,6-O-(2-thienylmethylene)-beta-D-glucopyranosyl]oxy]-furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one | [EINECS(EC#)]
249-831-2 | [Molecular Formula]
C32H32O13S | [MDL Number]
MFCD00866516 | [Molecular Weight]
656.65 | [MOL File]
29767-20-2.mol |
| Chemical Properties | Back Directory | [Melting point ]
244-247°C | [alpha ]
D20 -107° (9:1 chloroform/methanol) | [Boiling point ]
650.86°C (rough estimate) | [density ]
1.2568 (rough estimate) | [refractive index ]
1.5530 (estimate) | [storage temp. ]
-20°C | [solubility ]
DMSO: soluble10mg/mL, clear | [form ]
powder | [pka]
10.13(at 25℃) | [color ]
white to beige | [Optical Rotation]
[α]/D -100 to -115°, c = 1 in chloroform/methanol (9:1) | [λmax]
283nm(MeOH)(lit.) | [Merck ]
14,9145 | [Stability:]
Hygroscopic | [CAS DataBase Reference]
29767-20-2(CAS DataBase Reference) | [IARC]
2A (Vol. 76) 2000 |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
UN 2811 6.1 / PGIII | [WGK Germany ]
3 | [RTECS ]
KC0180000 | [HS Code ]
29349990 | [Safety Profile]
Poison by
intraperitoneal and subcutaneous routes. An
experimental teratogen. Human systemic
effects by ingestion and intravenous route:
anorexia, nausea or vomiting, leukopenia,
agranulocytosis and aplastic anemia of the
blood, bone marrow changes, and hair
changes. Experimental reproductive effects.
Human mutation data reported. When
heated to decomposition it emits very toxic fumes of SOx. | [Hazardous Substances Data]
29767-20-2(Hazardous Substances Data) | [Toxicity]
LD50 intraperitoneal in mouse: 29570ug/kg |
| Material Safety Data Sheet(MSDS) | Back Directory | [msds information]
[5R-[5alpha,5abeta,8aalpha,9beta(R*)]]-5,8,8a,9-Tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-9-[[4,6-O-(2-thienylmethylene)-beta-D-glucopyranosyl]oxy]-furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one(29767-20-2).msds |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Originator]
Vehem,Sandoz,France,1976 | [Uses]
A labelled semi-synthetic derivative of Podophyllotoxin. Antineoplastic. | [Uses]
A semi-synthetic derivative of Podophyllotoxin. Antineoplastic. | [Uses]
Teniposide is a podophyllotoxin derivative that causes dose-dependent single- and double-stranded breaks in DNA by inhibiting topoisomerase II. Its cytostatic effects are related to its ability to stabilize the DNA-topoisomerase II complex during DNA replication, inducing DNA damage and cellular apoptosis. Teniposide has been widely used in the treatment of various cancers including, small cell lung cancer, malignant lymphoma, breast cancer, oral squamous cell carcinoma, and acute lymphoblastic leukemia. | [Definition]
ChEBI: A furonaphthodioxole that is a synthetic derivative of podophyllotoxin with anti-tumour activity; causes single- and double-stranded breaks in DNA and DNA-protein cross-links and prevents repair by topoisomerase II binding. | [Indications]
Teniposide (VM-26, Vumon) is closely related to etoposide
in structure, mechanisms of action and resistance,
and adverse effects. It is more lipophilic and approximately
threefold more potent than etoposide. Its major
uses have been in pediatric cancers, particularly in acute
lymphoblastic leukemias. | [Manufacturing Process]
10 ml of pure thiophene-2-aldehyde and 0.25 g of anhydrous zinc chloride are American Home Products Corporation; British Patent 1,022,642; March 16,
1966
American Home Products Corporation; British Patent 1,022,645; March 16,
1966
Bell, S.C.; British Patent 1,057,492; February 1, 1967; assigned to American
Home Products Corporation | [Brand name]
Vumon (Bristol-Myers Squibb). | [Therapeutic Function]
Antineoplastic | [General Description]
Teniposide is available in 50-mg ampules with Cremophor ELfor IV administration in the treatment of acute lymphoblasticleukemia (ALL). The agent is more potent as an inhibitor oftopoisomerase II. The pharmacokinetics of teniposide issimilar to that of etoposide; however, the more lipophilic teniposideis more highly protein bound (99%) and less isexcreted unchanged in the urine (10%–20%). There is alsogreater overall metabolism of teniposide; however, CYP3A4-mediated conversion to the active catechol is slower comparedwith etoposide. Elimination occurs primarily in the urine witha terminal elimination half-life of 5 hours. | [Biochem/physiol Actions]
Teniposide (VM-26) is a Topoisomerase II inhibitor with antitumor activity. Teniposide inhibits DNA synthesis by forming a complex with topoisomerase II and DNA, inducing breaks in double stranded DNA and preventing repair. | [Clinical Use]
Teniposide is used in combination with other agents for the treatment of refractory childhood acute lymphoblastic leukemia. | [Synthesis]
Teniposide, [5R-(5|á,5a|?,8a|á,9|?)]-9-[4,6-O-(2-thienylmethylene)-|?-D-glucopy�ranosyl)oxy]- 5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3�,4�: 6,7]-naph�tho[2,3-d]-1,3-dioxol-6(5aH)-one (30.4.9), is basically synthesized by an analogous scheme
from 4�-benzyloxy-4�-desmethylepipodophyllotoxin (30.4.6), which is esterified by 2,3,4,6-
tetra-O-acetyl-|?-D-glucose in the presence of boron trifluoride etherate, giving a glycoside
30.4.7. The acetyl and benzyloxycarbonyl protecting groups in this molecule are removed by
succesive use of zinc acetate and sodium methoxide, and then by subsequent hydrogen reduc�tion, which forms the diol 30.4.8. The resulting diol is then transformed into the correspon�ding acetal 2-formylthiophene, which is the desired teniposide (30.4.9) .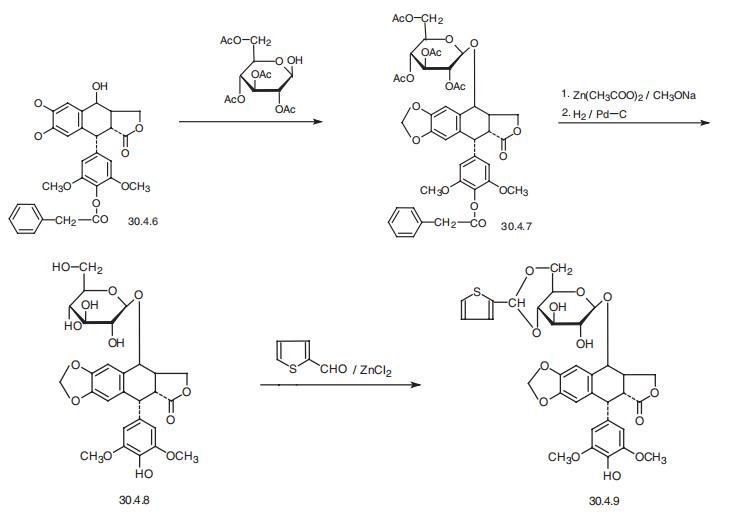
| [storage]
4°C, protect from light |
|
|