| Identification | More | [Name]
Chloroambucil | [CAS]
305-03-3 | [Synonyms]
4-[BIS(2-CHLOROETHYL)AMINO]BENZENEBUTANOIC ACID
4-[BIS(2-CHLOROETHYL)AMINO]BENZENEBUTYRIC ACID
4-[bis(2-chloroethyl)aminophenyl]butyric acid
4-[P-(BIS[2-CHLOROETHYL]AMINO)-PHENYL]BUTYRIC ACID
4-(p-(n,n-di-2-chloroethyl)aminophenyl)butyric acid
CHLORAMBUCIL
chloroambucil
3-[bis(2-chloroethyl)amino]benzenebutanoicacid
4-(4-[Bis(2-chloroethyl)amino]phenyl)butanoic acid
4-(bis(2-chloroethyl)amino)-benzenebutanoicaci
4-(bis(2-chloroethyl)amino)benzene-butanoicaci
4-(p-bis(2-chloroethyl)aminophenyl)-butyricaci
4-(p-Bis(beta-chloroethyl)aminophenyl)butyric acid
4(p-bis(beta-chloroethyl)aminophenyl)butyricacid
4-[Bis(2-chioroethyl)amino]benzenebutanoicacid
Ambochlorin
Amboclorin
Benzenebutanoic acid, 4-[bis(2-chloroethyl)amino]-
Butanoic acid, 4-(bis(2-chloroethyl)amino)benzene-
butanoicacid,4-[3-[bis(2-chloroethyl)amino]phenyl]- | [EINECS(EC#)]
206-162-0 | [Molecular Formula]
C14H19Cl2NO2 | [MDL Number]
MFCD00021783 | [Molecular Weight]
304.21 | [MOL File]
305-03-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Chlorambucil is a crystalline solid | [Melting point ]
65-70 °C
| [Boiling point ]
460.1±40.0 °C(Predicted) | [density ]
1.2486 (rough estimate) | [refractive index ]
1.6070 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Practically insoluble in water, freely soluble in acetone and in ethanol (96 per cent). | [form ]
neat | [pka]
pKa ~1.3(H2O) (Uncertain) | [color ]
White to Light yellow | [Stability:]
Stable, but may be light sensitive. Store cold. Incompatible with strong oxidizing agents. | [Water Solubility ]
<0.01 g/100 mL at 22 ºC | [λmax]
588nm(DMSO aq.)(lit.) | [Merck ]
13,2083 | [BRN ]
999011 | [BCS Class]
3/1 | [Major Application]
pharmaceutical (small molecule) | [InChI]
1S/C14H19Cl2NO2/c15-8-10-17(11-9-16)13-6-4-12(5-7-13)2-1-3-14(18)19/h4-7H,1-3,8-11H2,(H,18,19) | [InChIKey]
JCKYGMPEJWAADB-UHFFFAOYSA-N | [SMILES]
OC(=O)CCCc1ccc(cc1)N(CCCl)CCCl | [CAS DataBase Reference]
305-03-3(CAS DataBase Reference) | [IARC]
1 (Vol. 26, Sup 7, 100A) 2012 | [NIST Chemistry Reference]
Chlorambucil(305-03-3) | [EPA Substance Registry System]
305-03-3(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R45:May cause cancer.
R25:Toxic if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
ES7525000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29224999 | [Storage Class]
6.1C - Combustible acute toxic Cat.3
toxic compounds or compounds which causing chronic effects | [Hazard Classifications]
Acute Tox. 3 Oral
Carc. 1B
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 | [Safety Profile]
Confirmed carcinogen producing leukemia. Experimental
carcinogenic and neoplastigenic data. Poison
by ingestion, intravenous, intraperitoneal,
and subcutaneous routes. Human systemic
effects by ingestion: convulsions, cough,
dyspnea, and interstitial fibrosis. Human
reproductive effects by ingestion and
possibly other routes: changes in
spermatogenesis, menstrual cycle changes or
disorders, and teratogenic effects of the fetal
urogenital system. Experimental teratogenic
and reproductive effects. Human mutation
data reported. An anti-neoplastic agent.
When heated to decomposition it emits very
toxic fumes of Cland NOx. | [Hazardous Substances Data]
305-03-3(Hazardous Substances Data) | [Toxicity]
LD50 i.p. in rats: 58.2 mmole/kg (Ross) |
| Hazard Information | Back Directory | [General Description]
White to pale beige crystalline or granular powder with a slight odor. Melting point 65-69°C. | [Reactivity Profile]
CHLORAMBUCIL(305-03-3) is an alkylating agent. Reacts with proteins and a variety of nucleophilic compounds . | [Air & Water Reactions]
Insoluble in water. | [Potential Exposure]
Chlorambucil, an anticancer drug, is a
derivative of nitrogen mustard. This drug is primarily used
as an antineoplastic agent for treating lymphocytic leukemia; malignant lymphomas; follicular lymphoma; and
Hodgkin’s disease. The treatments are not curative but do
produce some marked remissions. Chlorambucil has also
been tested for treatment of chronic hepatitis, rheumatoid
arthritis; and as an insect chemosterilant. All of the chemical used in this country is imported from the United
Kingdom. Work exposure in the United States would be
limited to workers formulating the tablets, or to those
patients receiving the drug. | [Fire Hazard]
Literature sources indicate that this chemical is nonflammable. | [First aid]
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; give artificial respiration with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin contact,
avoid spreading material on unaffected skin. Keep victim
warm and quiet. Effects of exposure (inhalation, ingestion,
or skin contact) to substance may be delayed. Ensure that
medical personnel are aware of the material(s) involved
and take precautions to protect themselves. Medical observation is recommended for 24 to 48 hours after breathing
overexposure, as pulmonary edema may be delayed. As
first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a drug or other inhalation therapy. | [Shipping]
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. | [Incompatibilities]
Moisture. Chlorambucil is an alkylating
agent. Reacts with proteins and a variety of nucleophilic
compounds. Compounds of the carboxyl group react
with all bases, both inorganic and organic (i.e., amines)
releasing substantial heat, water, and a salt that may beharmful. Incompatible with arsenic compounds (releases
hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, sulfides (releasing
heat, toxic, and possibly flammable gases), thiosulfates,
and dithionites (releasing hydrogen sulfate and oxides of
sulfur). | [Description]
Chlorambucil, approved by the Food and Drug Administration
(FDA) in 1957, is an antineoplastic/alkylating agent with
a broad spectrum of antitumor activity used to treat chronic
lymphocytic leukemia (CLL), Hodgkin’s and non-Hodgkin’s
lymphomas. | [Chemical Properties]
beige powder | [Chemical Properties]
Chlorambucil is a crystalline solid | [Waste Disposal]
It is inappropriate and possibly dangerous to the environment to dispose of expired or
waste drugs and pharmaceuticals by flushing them down
the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed
with wet cat litter or coffee grounds, double-bagged in
plastic, discard in trash. Larger quantities shall carefully
take into consideration applicable DEA, EPA, and FDA
regulations. If possible return the pharmaceutical to the
manufacturer for proper disposal being careful to properlylabel and securely package the material. Alternatively, the
waste pharmaceutical shall be labeled, securely packaged,
and transported by a state licensed medical waste contractor
to dispose by burial in a licensed hazardous or toxic waste
landfill or incinerator. Permanganate oxidation, high temperature incineration with scrubbing equipment, or microwave plasma treatment. | [Originator]
Leukeran,Burroughs�Wellcome,US,1957 | [Uses]
antineoplastic, alkylating agent | [Uses]
Chlorambucil is a alkylating agent that is used as an chemotherapy drug in the treatment of chronic lymphocytic leukemia. Chlorambucil is also used to treat non-Hodgkin's lymphoma (NHL) and Hodgkin's
disease. | [Uses]
Chlorambucil-d8 is the isotope labelled analogue of Chlorambucil (C324050), an alkylating agent that is used in the treatment of chronic lymphocytic leukemia. Chlorambucil is also used to treat non-Ho
dgkin's lymphoma (NHL) and Hodgkin's disease. | [Uses]
tranquilization aid | [Definition]
ChEBI: A monocarboxylic acid that is butanoic acid substituted at position 4 by a 4-[bis(2-chloroethyl)amino]phenyl group. A chemotherapy drug that can be used in combination with the antibody obinutuzumab for the treatment of chronic lymphocytic leukemia. | [Indications]
Chlorambucil (Leukeran) is an aromatic nitrogen mustard
that is intermediate in chemical reactivity between
mechlorethamine and melphalan. Its mechanisms of action
and range of antitumor activity are similar to theirs.
It is well absorbed orally, but detailed information concerning
its metabolic fate in humans is lacking.
Chlorambucil is used primarily as daily palliative
therapy for chronic lymphocytic leukemia, Waldenstr?om’s
macroglobulinemia, myeloma, and other lymphomas.
Bone marrow toxicity is the major side effect of
chlorambucil. Nausea is uncommon or mild, and hair
loss does not occur. Chlorambucil shares the immunosuppressive,
teratogenic, and carcinogenic properties of
the nitrogen mustards. | [Manufacturing Process]
Acetanilide and maleic acid are condensed to give beta-(p-acetaminobenzoyl)
acrilic acid which is hydrogenated to give methyl-gamma-(p-aminophenyl)
butyrate. That is reacted with ethylene oxide and then with phosphorus
oxychloride to give the methyl ester which is finally hydrolyzed to give
chlorambucil. | [Brand name]
Leukeran
(GlaxoSmithKline. | [Therapeutic Function]
Antineoplastic | [Biochem/physiol Actions]
Chlorambucil is an anti-cancer drug that alkylates DNA and induces apoptosis. Death of chronic lymphocytic leukemia cells occurs via a p53-dependent mechanism. | [Clinical Use]
It is used in the palliative treatment of chronic lymphocytic leukemia,
malignant lymphoma, and Hodgkin's disease. | [Synthesis]
Chlorambucil, 4-[p-[bis-(2-chloroethyl)amino]phenyl]butyric acid (30.2.1.7),
is made from acetanilide and succinic anhydride. In the first stage of synthesis, acetanilide is
acylated by succinic anhydride, giving 4-(4-acetaminophenyl)-4-ketobutyric acid (30.2.1.3).
The keto group in this compound is reduced by hydrogen in a methanol solution using palla�dium on carbon as a catalyst. This results in the formation of the methyl ester of 4-(4-aceta�minophenyl)-butyric acid (30.2.1.4). This is treated with an alkali in order to hydrolyze both
the amide and ester parts of the molecule, which forms 4-(4-aminophenyl)butyric acid
(30.2.1.5), which upon reaction with ethylene oxide gives 4-[p-[bis(2-hydroxyethyl)
amino]phenyl]butyric acid (30.2.1.7). Replacing all of the hydroxyl groups in this compound
using phosphoryl chloride and subsequent treatment with water to hydrolyze the resulting
intermediate acid chloride to an acid gives chlorambucil (30.2.1.7).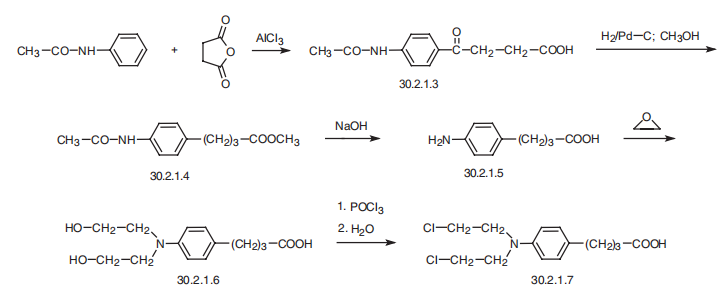
| [Veterinary Drugs and Treatments]
Chlorambucil may be useful in a variety of neoplastic diseases, including
lymphocytic
leukemia, multiple myeloma, polycythemia
vera, macroglobulinemia, and ovarian adenocarcinoma.
It may also
be useful as adjunctive therapy for some immune-mediated conditions
(e.g., glomerulonephritis, inflammatory bowel disease, nonerosive
arthritis, or immune-mediated skin disease). It has found
favor as a routine treatment for feline pemphigus foliaceous and
severe feline eosinophilic granuloma complex due to the drug’s relative
lack of toxicity in cats and efficacy. | [Drug interactions]
Potentially hazardous interactions with other drugs
Ciclosporin: ciclosporin concentration possibly
reduced.
Patients who receive phenylbutazone may require
reduced doses of chlorambucil. | [Carcinogenicity]
Chlorambucil is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans. | [Environmental Fate]
The mechanism of action of chlorambucil is thought to be an
alkylating agent and an aromatic nitrogen mustard derivative; it
interferes with DNA replication and RNA transcription by
alkylation and cross-linking the strands of DNA. | [Metabolism]
Chlorambucil is extensively metabolised in the liver
via the hepatic microsomal enzyme oxidation system,
principally to phenylacetic acid mustard, which is
pharmacologically active, and which also undergoes some
spontaneous degradation to further derivatives.
Chlorambucil is excreted in the urine, almost exclusively
as metabolites with less than 1% unchanged. | [storage]
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with chlorambucil you should be trained on its proper handling and storage. Store in cool, dry place. Store in sealed ampules or inamber screw-capped bottles or vials with Teflon? capliners. Solutions may be stored in bottles or vials with a silicone system having a Teflon? liner and sampled with needle and syringe. Prevent exposure to light. A regulated,marked area should be established where this chemical ishandled, used, or stored in compliance with OSHAStandard 1910.1045. | [Purification Methods]
Chlorambucil is recrystallised from pet ether (flat needles) and has a solubility at 20o of 66% in EtOH, 40% in CHCl3, 50% in Me2CO but is insoluble in H2O [Everett et al. J Chem Soc 2386 1953]. [Beilstein 14 IV 1715.] CARCINOGEN. | [Toxicity evaluation]
The chemical is of a white to pale slight odorous powder,
insoluble in water. It is very slightly dispersible in diethyl ether
and acetone. It has a melting point of 69°C, boiling point of
424°C, and 5.75 pKa. The partition coefficient is 4.07 and has
a molecular weight of 304.22 g mol�-1. |
|
|