| Identification | More | [Name]
Carmustin | [CAS]
154-93-8 | [Synonyms]
1,3-BIS(2-CHLOROETHYL)-1-NITROSOUREA
1,3-BIS(2-CHLOROETHYL)NITROSOUREA
BCNU
BECENUN
BICNU
bis(2-chloroethyl)nitrosourea
CARMUSTIN
CARMUSTINE
N,N'-BIS(2-CHLOROETHYL)-N-NITROSOUREA
NSC-409962
1,3-bis(2-chloroethyl)-1-nitroso-ure
1,3-bis(beta-chloroethyl)-1-nitrosourea
bischlorethylnitrosurea
bischloroethylnitrosourea
carmubris
fda0345
n,n’-bis(2-chloroethyl)-n-nitroso-ure
nci-c04773
nitrumon
sk27702 | [EINECS(EC#)]
205-838-2 | [Molecular Formula]
C5H9Cl2N3O2 | [MDL Number]
MFCD00057706 | [Molecular Weight]
214.05 | [MOL File]
154-93-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Carmustine is an orange-yellow crystalline
solid or powder. | [Melting point ]
30 °C (lit.) | [density ]
1.6948 (rough estimate) | [refractive index ]
1.6100 (estimate) | [storage temp. ]
−20°C
| [solubility ]
insoluble in H2O; ≥21.51 mg/mL in DMSO; ≥27.15 mg/mL in EtOH | [form ]
(Oily liquid to amorphous solid)
| [pka]
10.19±0.46(Predicted) | [color ]
Light-yellow powder | [Water Solubility ]
<0.1 g/100 mL at 18 ºC | [Usage]
An alkylating and carbamoylating nitrosourea compound. It interacts with DNA, RNA and proteins causing DNA interstrand cross linking which is cytotoxic and leads to apoptotic cell death | [Merck ]
14,1845 | [Stability:]
Temperature Sensitive | [InChIKey]
DLGOEMSEDOSKAD-UHFFFAOYSA-N | [CAS DataBase Reference]
154-93-8(CAS DataBase Reference) | [IARC]
2A (Vol. 26, Sup 7) 1987 | [EPA Substance Registry System]
154-93-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T+ | [Risk Statements ]
R45:May cause cancer.
R60:May impair fertility.
R61:May cause harm to the unborn child.
R28:Very Toxic if swallowed. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
YS2625000
| [HazardClass ]
6.1(a) | [PackingGroup ]
II | [HS Code ]
29241990 | [Hazardous Substances Data]
154-93-8(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 19-25 orally, 26 i.p., 24 s.c.; in rats (mg/kg): 30-34 orally (Thompson, Larson) |
| Hazard Information | Back Directory | [General Description]
Orange-yellow solid. | [Reactivity Profile]
1,3-BIS(2-CHLOROETHYL)-1-NITROSOUREA(154-93-8) decomposes rapidly in acid and in solutions above pH 7; most stable in petroleum ether or aqueous solution at pH 4. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Extremely toxic, central nervous system
depression, pulmonary fibrosis, renal and hepatic
damage, cytotoxic, immunosuppressive, carcino-
gen.
| [Potential Exposure]
BCNU has been used since 1971 as an
antineoplastic agent in the treatment of Hodgkin’slymphoma; multiple meyloma; and primary or metastatic
brain tumors. It also has been reported to have antiviral,
antibacterial, and antifungal activity, but no evidence was
found that it is used in these ways. BCNU is not known to
be naturally occurring. Health professionals who handle
this drug (for example, pharmacists, nurses, and physicians)
may possibly be exposed to BCNU during drug preparation, administration, or cleanup; however, the risks can be
avoided through use of containment equipment and proper
work practices | [Fire Hazard]
Flash point data for this chemical are not available. 1,3-BIS(2-CHLOROETHYL)-1-NITROSOUREA is probably combustible. | [First aid]
Skin Contact: Flood all areas of body that have
contacted the substance with water. Don’t wait to remove
contaminated clothing; do it under the water stream. Use
soap to help assure removal. Isolate contaminated clothing when removed to prevent contact by others. Eye
Contact: Remove any contact lenses at once. Immediately
flush eyes well with copious quantities of water or normal
saline for at least 20�30 minutes. Seek medical attention.
Inhalation: Leave contaminated area immediately;
breathe fresh air. Proper respiratory protection must be
supplied to any rescuers. If coughing, difficult breathing,
or any other symptoms develop, seek medical attention at
once, even if symptoms develop many hours after exposure. Ingestion: Contact a physician, hospital, or poison
center at once. If the victim is unconscious or convulsing,
do not induce vomiting or give anything by mouth.
Assure that the patient’s airway is open and lay him on
his side with his head lower than his body and transport
immediately to a medical facility. If conscious and not
convulsing, give a glass of water to dilute the substance.Vomiting should not be induced without a physician’s
advice. | [Shipping]
UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic
solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-
Poisonous materials, Technical Name Required. | [Incompatibilities]
Acids and acid solutions above pH 7
cause rapid decomposition. Most stable at pH 4 in aqueous
solution or petroleum ether. | [Description]
Bischloroethyl nitrosourea (BCNU) is a mustard-gas-derived
alkylating agent that underwent clinical trials for use as an
antineoplastic agent in the mid-1960s. Intravenous BCNU
received US Food and Drug Administration (FDA) approval for
brain tumor treatments in 1977. Further development and
trials led to the FDA approval of a BCNU-impregnated polymer
wafer for use as an intracavity surgical adjunct for recurrent
glioblastoma moltiforme in 1996. These wafers were again
reapproved in 2003 for use in high-grade malignant glioma as
an adjunct to surgery and radiation. | [Waste Disposal]
It is inappropriate and possibly dangerous to the environment to dispose of expired or
waste pharmaceuticals by flushing them down the toilet or
discarding them to the trash. Household quantities of
expired or waste pharmaceuticals may be mixed with wet
cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If
possible return the pharmaceutical to the manufacturer for
proper disposal being careful to properly label and securely
package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a
state licensed medical waste contractor to dispose by burial
in a licensed hazardous or toxic waste landfill or
incinerator. | [Originator]
BCNU,Gencorp Aerojet,US | [Definition]
ChEBI: A member of the class of N-nitrosoureas that is 1,3-bis(2-chloroethyl)urea in which one of the nitrogens is substituted by a nitroso group. | [Manufacturing Process]
A solution of sodium nitrite (6.9 g, 0.10 mole) in water (60 ml) was added
dropwise to a cold (0-5°C), stirred solution of 1,3-bis(2-chloroethyl)urea (8.0
g, 0.044 mole) in formic acid (50 ml). The reaction mixture was stirred further
at 0°C until the pale yellow oil that had formed solidified. The nitrosourea was
collected and washed quickly with cold water (2 x 10 ml), and dried in
vacuum; yield 6.7 g. (71%). | [Brand name]
Bicnu (Bristol-Myers Squibb); Gliadel
(Millot Laboratories, France). | [Therapeutic Function]
Antitumor | [Biochem/physiol Actions]
Carmustine is a DNA alkylating agent causing DNA interstrand crosslinks. Effective against glioma and other solid tumors. | [Clinical Use]
Alkylating agent:
Myeloma, lymphoma and brain tumours | [Safety Profile]
Confirmed carcinogen withexperimental carcinogenic and tumorigenic data. A humanpoison by parenteral route. An experimental poison byingestion, intravenous, intraperitoneal, parenteral, andsubcutaneous routes. Human systemic effects byparenteral, int | [Synthesis]
Carmustin, 1,3-bis-(2-chloroethyl)-1-nitrosourea (30.2.4.4), is made by
nitrating 1,3-bis(2-chloroethyl)urea with nitrogen trioxide.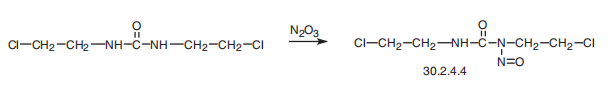
| [Drug interactions]
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis). | [Carcinogenicity]
Bis(chloroethyl) nitrosourea (BCNU) is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | [Environmental Fate]
It is generally assumed that BCNU exerts its cytotoxicity
through the liberation of alkylating and carbamoylating
moieties. An alkylating entity, particularly chloroethyl carbonium
ion, is strongly electrophilic and can alkylate a variety
of biomolecules, including the purine and pyrimidine bases of
DNA. BCNU causes DNA interstrand cross-linking, which is
associated with cytotoxicity. The carbamoylation of lysine
residues of protein can inactivate certain enzymes, thus interfering
with DNA and RNA synthesis and repair processes. The
inhibition of glutathione reductase by this carbamoylation
further contributes to cytotoxicity. | [Metabolism]
Intravenous carmustine is rapidly metabolised, and no
intact drug is detectable after 15 minutes. It is partially
metabolised to active metabolites by liver microsomal
enzymes, which have a long half-life. It is thought that the
antineoplastic activity may be due to metabolites.
Approximately 30% of a dose is excreted in the urine after
24 hours, and 60-70% of the total dose after 96 hours.
About 10% is excreted as respiratory CO2
. Terminal half�life of the metabolites is about 1 hour. | [storage]
-20°C, protect from light | [Toxicity evaluation]
There is no information available on the environmental fate of
BCNU. However, it is predicted that BCNU spontaneously decomposes due to its high reactivity. Estimates indicate that
the half-life of BCNU particulates and vapor in air is 4.4 days.
Though expected to be highly mobile when adsorbed to soil
and suspended solids, it is likely that this adsorption may be
precluded by hydrolysis. Volatilization from soil or water is not
expected, and the potential for bioaccumulation is low. BCNU
degrades into 2-chloroethylamine, which is not considered
hazardous to the environment. | [References]
[1] TODD H. WASSERMAN MD Milan S M, PHD Stephen K C M. Clinical comparison of the nitrosoureas[J]. Cancer, 1975, 36 4: 1258-1268. DOI: 10.1002/1097-0142(197510)36:4<1258::aid-cncr2820360411>3.0.co;2-6
[2] M R SOMA. In vivo enhanced antitumor activity of carmustine [N,N’-bis(2-chloroethyl)-N-nitrosourea] by simvastatin.[J]. Cancer research, 1995, 55 3: 597-602.
[3] CRYSTAL D HAYES. Striking reduction of amyloid plaque burden in an Alzheimer’s mouse model after chronic administration of carmustine.[J]. BMC Medicine, 2013, 11: 81. DOI: 10.1186/1741-7015-11-81 |
| Questions And Answer | Back Directory | [Cyclically non-specific anti-tumor drug]
It appears as colorless or yellowish or yellowish green crystal or crystalline powder and is odorless. It is insoluble in water and soluble in methanol or ethanol. Its hydrous solution is stable at pH4 but will be subject to rapid decomposition at solution of pH above 7.
Carmustin, together with lomustine, fotemustine and semustine are currently the most widely used cyclically non-specific anti-tumor drugs. It belongs to nitrosourea alkylating agent, although with the role of alkylating agents, has no cross-resistance with general alkylating agent. It is characterized by high lipid solubility, broad anti-tumor spectrum the alkylating agent is generally no cross-resistance with high fat-soluble, broad spectrum anti-tumor, quick onset and easily penetrating through the blood-brain barrier and so on. In the body, it can be decomposed into two active ingredients with one having carbamoyl activity and the other being as alkylating agent that can react with the DNA polymerase to inhibit the synthesis of RNA and DNA. It has effect on the proliferation of cells in each stage while being insensitive to non-proliferating cells. It is easily absorbed orally. It enters the brain at one hour after the intravenous administration. At six hours after administration, the brain drug concentration can reach about 60% to 70% of the plasma concentration with in vivo distribution being the highest in the liver, bile, kidney and spleen. This product has a short half-life being less than 15 minutes. But its metabolites have long half-life, and still have anti-cancer effects with being slowly released after binding to the plasma protein. Therefore, its effect can last long and produce delayed toxicity. This product is rapidly metabolized in the blood after being absorbed with the metabolites excreted slowly and the plasma concentration still remaining high after 48 hours. 60% is excreted through urine in the form of metabolites.
It is commonly used in the treatment of primary and secondary brain cancer, Hodgkin's disease, meningeal leukemia. It can also be applied for the treatment of multiple myeloma, lymphoma, breast cancer, lymphoma, melanoma, lung cancer; combination with fluorouracil can be adopted for treating colorectal cancer and gastric cancer; it can be used for treating bronchus lung cancer when being used in combination with methotrexate and cyclophosphamide. Carmustin is also effective in treating cancer of head portion as well as testicular cancer.
| [Toxic reaction]
1, bone marrow suppression: it is dose-limiting toxicity, exhibiting as severe neutropenia and thrombocytopenia, usually occurs at 3 to 5 weeks after administration and will last for 1 to 3 weeks with the lowest suppression point occurring in 3 to 5 weeks with the ease being slowly than other alkylating agents.
2, gastrointestinal reactions: severe nausea, vomiting usually begins two hours after administration and will last for 4 to 6 hours. Administration of antiemetic agent before the treatment can prevent this.
3 Other reactions: burning sensation can immediately happen at injection site and limbs. Rare toxicity including liver and kidney dysfunction, usually occur upon large doses administration. It has been reported of the occurrence of painless jaundice and hepatic coma as well as pulmonary fibrosis.
| [Carmustin]
Carmustin belongs to nitrosourea alkylating agents. On the one hand, it binds to DNA through alkylation. On the other hand, it acts on the protein through carbamoylation. It can inhibit DNA polymerase, thus preventing DNA and RNA synthesis with the strongest effect on the G1-s transition period as well as blocking effect on the s-phase, and further enhanced effect on the G2 phase and also certain effect on the G0 phase. It is a cell-cycle non-specific drug. This product high an excellent lipid-solubility, low dissociation and can penetrate through the blood-brain barrier with its metabolites still having anti-cancer effects. It undergoes slow release after binding to protein, thus having a long-lasting efficacy. It has broad anti-tumor spectrum with excellent efficacy in the treatment of meningeal leukemia, brain and spinal cord metastasis of malignant tumors, Hodgkin's disease as well as acute leukemia. It also has certain efficacy on the treatment of breast cancer, lung cancer, bone metastasis, lymphatic sarcoma, melanoma and testicular cancer. It is effective for treating primary and secondary brain tumors. Topical administration has excellent efficacy in treating lymphoma papules. The drug, in combination with fluorouracil, vincristine, dacarbazine, consists FIVB protocol for the treatment of colon; together with fluorouracil and doxorubicin, it form FAB protocol for treating gastric cancer; in combination with vincristine and dacarbazine, it can be used for the treatment of melanoma; in combination with androgen, it can be used for the therapy of breast cancer.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| [Chemical Properties]
It appears as slightly yellow crystalline powder with the melting point being 30-32 ℃ and becoming oily liquid after melting. It is soluble in methanol, ethanol with a solubility in 50% ethanol being 150mg/ml and the water solubility being 4 mg/ml. It is mostly stable in the aqueous solution of pH4 and petroleum ether.
| [Uses]
The product is a broad-spectrum anti-cancer drug with excellent efficacy for the treatment of acute leukemia and Hodgkin's disease as well certain efficacy on the treatment of breast cancer, lung cancer and brain cancer as well as the bone metastases of cancer as well. Oral administration for mouse has a LD50 of 19-25mg/kg while the value for intraperitoneal injection is 26mg/kg, 24 mg/kg for subcutaneous injection; rat which is subject to oral administration has a LD50 of 30-40mg/kg.
| [Production method]
The product has three synthetic routes: 1. take ethylene imine as raw material, go through phosgene condensation to generate bis-(β-chloroethyl) urea, and then generate carmustin via nitrosation; 2.take urea as raw material, go through condensation, ring-opening, chlorination, nitrosation to obtain it; 3.take ethanolamine as raw materials, and generate carmustin through similar processes as methods2. The first method can generate the finished product with just two steps but with its raw material, phosgene and ethyleneimine, both being extremely toxic chemicals, therefore demanding a high-level labor protection and production equipment. The second method has readily available raw materials as well as convenient operation.
| [Category]
Toxic substances
| [Toxicity grading]
Highly toxic
| [Acute toxicity]
Oral-rat LD50: 20 mg/kg; Oral-Mouse LD50: 19 mg/kg.
| [Hazardous characteristics of explosive]
It may cause deadly harm to the human respiratory system and can cause pulmonary fibrosis, dyspnea and verticillium.
| [Flammability and hazard characteristics]
Combustion can produce toxic nitrogen oxides, chlorides fumes; it can lead to poisoning: nausea, vomiting, leukopenia and thrombocytopenia as well as bone marrow damage.
| [Storage characteristics]
Treasury: ventilation, low-temperature and dry; store it separately from food raw materials.
| [Extinguishing agent]
Dry powder, foam, sand, carbon dioxide, water mist. |
|
|