| Identification | More | [Name]
Metoprolol | [CAS]
37350-58-6 | [Synonyms]
METOPROLOL
1-(Isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]-2-propanol
1-(Isopropylamino)-3-[p-(2-methoxyethyl)phenoxy]-2-propanol
2-Propanol, 1-[4-(2-methoxyethyl)phenoxy]-3-[(1-methylethyl)amino]-
2-Propanol, 1-[4-(2-methoxyethyl)phenoxy]-3-[(1-methylethyl)amino]-, (±
2-Propanol, 1-[4-(2-methoxyethyl)phenoxy]-3-[(1-methylethyl)amino]-, (.+/-.)-
CGP 2175
cgp2175
H-23/96
Lopresoretic
Metoprolol (base and/or unspecified salts)
1-[4-(2-methoxyethyl)phenoxy]-3-propan-2-ylamino-propan-2-ol
Metoprolol
Metroprolol
(±)-Metoprorol
Methoprorol
Metoprorol | [EINECS(EC#)]
253-483-7 | [Molecular Formula]
C15H25NO3 | [MDL Number]
MFCD00599534 | [Molecular Weight]
267.36 | [MOL File]
37350-58-6.mol |
| Hazard Information | Back Directory | [Originator]
Betaloc,Astra,UK,1975 | [Uses]
Antiadrenergic (β-receptor). | [Uses]
Metoprolol is a β-adrenergic blocking antihypertensive and antianginal agent; to treat raised blood pressure; to prevent attacks of angina (pain
from an inadequate oxygen supply to the heart); after a heart attack to prevent further damage to the heart muscle; treatment of some
disturbances of heart rhythm; used to help prevent attacks of migraine; in ophthalmic preparations; treatment of myocardial infarcts. | [Uses]
Metoprolol is used in moderate hypertension, serious conditions of myocardial infarc�tion, for preventing death of cardiovascular tissue, in angina, tachycardia, extrasystole, and
for secondary prophylaxis after a heart attack. | [Definition]
ChEBI: Metoprolol is a propanolamine that is 1-(propan-2-ylamino)propan-2-ol substituted by a 4-(2-methoxyethyl)phenoxy group at position 1. It has a role as a beta-adrenergic antagonist, an antihypertensive agent, a xenobiotic, an environmental contaminant and a geroprotector. It is a propanolamine, an aromatic ether, a secondary alcohol and a secondary amino compound. | [Manufacturing Process]
The starting material 1,2-epoxy-3-[p-(β-methoxyethyl)phenoxy]-propane was
obtained from p-(β-methoxyethyl)-phenol which was reacted with
epichlorohydrin whereafter the reaction product was distilled at 118°C to
128°C at a pressure of 0.35mm Hg.
1,2-Epoxy-3-[p-(β-methoxyethyl)-phenoxy]-propane (16.7g) was dissolved in
50 ml isopropanol and mixed with 20 ml isopropylamine. The mixture was
heated in an autoclave on boiling water-bath overnight, whereafter it was
evaporated and the remainder dissolved in 2 N HCI. The solution was
extracted first with ether and thereafter with methylene chloride. After
evaporating the methylene chloride phase, the hydrochloride of 1-
isopropylamino-3-[p(β-methoxyethyl)-phenoxy] -propanol-2 was obtained
which, after recrystallization from ethyl acetate, weighed 10.4 g. Melting point
83°C. Equivalent weight: found 304.0, calculated 303.8.
The hydrochloride is then converted to the tartrate. | [Brand name]
Lopressor (Novartis). | [Therapeutic Function]
Beta-adrenergic blocker | [Mechanism of action]
Unlike propranolol, which blocks both β1 and β2-adrenoreceptors, metroprolol exhibits
cardioselective action, i.e. in therapeutic doses, it blocks β1-adrenoreceptors with insignificant
effects on β2-adrenoreceptors. | [Synthesis]
Metoprolol, 1-(iso-propylamino)-3-[4??(2-methoxyethyl)phenoxy]-2-
propanol (12.1.5), is synthesized by reacting 4-(2-methoxyethyl)phenol with epichlorhy�dride in the presence of a base, isolating 1,2-epoxy-3-[4??(2-methoxyethyl)phenoxy]
propane (12.1.4), the subsequent reaction of which, analogous to that described before,
with iso-propylamine, gives an opening of the epoxide ring and leads to the formation of
metoprolol (12.1.5) [7,8].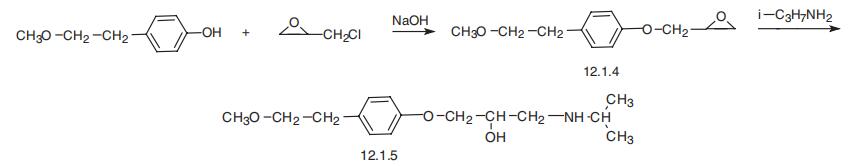
| [Metabolism]
The pharmacokinetic profile of metoprolol (Lopressor)
is similar to that of propranolol. Metoprolol is readily
and rapidly absorbed after oral administration and is
subject to a significant amount of first-pass metabolism
by the liver. Curiously, the duration of metoprolol’s action
is longer than one would predict from its plasma
half-life, which ranges from 0.5 to 2.5 hours. The degree
of binding of metoprolol to plasma proteins is modest
(10%). The extensive distribution of metoprolol to the
lungs and kidney is typical of a moderately lipophilic
drug. Metoprolol undergoes considerable metabolism;only 3 to 10% of an administered dose is recovered as
unchanged drug.The metabolites are essentially inactive
as -receptor blocking agents and are eliminated primarily
by renal excretion. Small amounts of the drug are
present in the feces. |
|
|