| Identification | More | [Name]
Nonafluorobutanesulfonyl fluoride | [CAS]
375-72-4 | [Synonyms]
NONAFLUORO-1-BUTANESULFONYL FLUORIDE
NONAFLUOROBUTANESULFONYL FLUORIDE
NONAFLUOROBUTANESULPHONYL FLUORIDE
PERFLUORO-1-BUTANESULFONYL FLUORIDE
PERFLUOROBUTANESULFONYL FLUORIDE
PERFLUOROBUTANESULPHONYL FLUORIDE
1,1,2,2,3,3,4,4,4-nonafluoro-1-butanesulfonylfluorid
1,1,2,2,3,3,4,4,4-Nonafluoro-1-butanesulfonylfluoride
1-Butanesulfonyl fluoride, 1,1,2,2,3,3,4,4,4-nonafluoro-
1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulphonyl fluoride
Perfluorobutansulfonylfluoride
Nonaflylfluoride
Perfluoro n-butylsulfonyl fluoride
Perfluorobutansulfonylfluoride-RM60
Nonafluorobutanesulphonyl fluoride 95%
Nonafluorobutanesulphonylfluoride95%
Nonafluorobutanesulfonyl fluoride, 90+%
FC-4
Nonafluorobutanesulphonyl fluoride, tech. 90%
1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulfonyl fluoride | [EINECS(EC#)]
206-792-6 | [Molecular Formula]
C4F10O2S | [MDL Number]
MFCD00007422 | [Molecular Weight]
302.09 | [MOL File]
375-72-4.mol |
| Chemical Properties | Back Directory | [Appearance]
CLEAR COLORLESS LIQUID | [Melting point ]
-110°C | [Boiling point ]
64 °C (lit.) | [density ]
1.716 g/mL at 20 °C(lit.)
| [vapor pressure ]
16.665kPa at 20℃ | [refractive index ]
n20/D 1.3(lit.)
| [Fp ]
65-66°C | [storage temp. ]
Keep in dark place,Sealed in dry,Store in freezer, under -20°C | [solubility ]
Chloroform (Sparingly), Methanol (Sparingly) | [form ]
Liquid | [color ]
Colorless to yellow | [Specific Gravity]
1.682 | [Water Solubility ]
Hydrolyses in water. | [Sensitive ]
Moisture Sensitive | [BRN ]
1813589 | [Stability:]
Hygroscopic | [InChI]
1S/C4F10O2S/c5-1(6,3(9,10)11)2(7,8)4(12,13)17(14,15)16 | [InChIKey]
LUYQYZLEHLTPBH-UHFFFAOYSA-N | [SMILES]
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)S(F)(=O)=O | [CAS DataBase Reference]
375-72-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Nonafluorobutanesulfonyl fluoride(375-72-4) | [EPA Substance Registry System]
375-72-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S25:Avoid contact with eyes . | [RIDADR ]
UN 3265 8/PG 2
| [WGK Germany ]
3
| [F ]
10-21 | [Hazard Note ]
Corrosive | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29049090 | [Storage Class]
8A - Combustible corrosive hazardous materials | [Hazard Classifications]
Skin Corr. 1B |
| Hazard Information | Back Directory | [Description]
1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulfonyl fluoride
(NfF,375-72-4) also called Nonafluorobutanesulfonyl fluoride is a versatile compound in organic synthesis. It can be
used as a fluoride source for the nucleophilic introduction
of fluorine, but it is also frequently applied as sulfonylation
reagent generating intermediates with strong electron withdrawing perfluorinated alkyl substituents.
| [Chemical Properties]
CLEAR COLORLESS LIQUID | [Uses]
Benzynes were generated from o-(trimethylsilyl) phenols using nonafluorobutanesulfonyl fluoride (NfF) by a domino process.Nonafluorobutanesulfonyl fluoride (nonaflyl fluoride, C 4 F 9 SO 2 F, NfF) is the most widely used commercially available reagent for the synthesis of nonaflates. | [Application]
Perfluoro-1-butanesulfonyl fluoride (NfF,375-72-4) reacts with alcohols, including phenols, to yield nonafluorobutanesulfonate esters (nonaflates). Nonaflates can be used as electrophiles in several palladium-catalyzed cross coupling reactions and in Buchwald-Hartwig amination. NfF can also be used to prepare aryl nonaflates by reacting with corresponding aryloxysilanes in the presence of fluoride ion.
| [reaction suitability]
reaction type: click chemistry | [Synthesis]
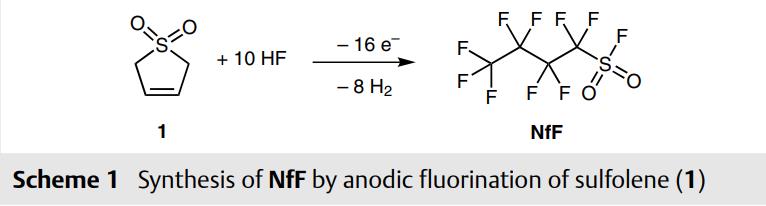
NfF(Nonafluorobutanesulfonyl fluoride,375-72-4) is produced on industrial scale by anodic fluorination of sulfolene (1, Scheme 1),3 therefore it is a fairly cheap reagent and commercially available from several suppliers. The compound is bench-stable and storable for years, nontoxic and easy to handle.
| [storage]
Store in amber colored bottles protected from light; for prolonged storage, PVC bottles are recommended. | [References]
[1] JOSE LUIS CHIARA; José R S. Synthesis of α-Diazo Carbonyl Compounds with the Shelf-Stable Diazo Transfer Reagent Nonafluorobutanesulfonyl Azide[J]. Advanced Synthesis & Catalysis, 2011. DOI:10.1002/adsc.201000846.
[2] YU TANG; Biao Y; D Prabhakar Reddy. A dehydrative glycosylation protocol mediated by nonafluorobutanesulfonyl fluoride (NfF)[J]. Tetrahedron, 2021. DOI:10.1016/j.tet.2020.131800. |
|
|