| Identification | More | [Name]
Gabapentin enacarbil | [CAS]
478296-72-9 | [Synonyms]
GABAPENTIN ENACARBIL
1-[[[[1-(2-Methyl-1-oxopropoxy)ethoxy]carbonyl]amino]methyl]cyclohexaneacetic acid | [Molecular Formula]
C16H27NO6 | [MDL Number]
MFCD09954124 | [Molecular Weight]
329.39 | [MOL File]
478296-72-9.mol |
| Chemical Properties | Back Directory | [Melting point ]
65 °C | [Boiling point ]
482.0±20.0 °C(Predicted) | [density ]
1.134 | [storage temp. ]
Store at -20°C | [solubility ]
DMSO:100.0(Max Conc. mg/mL);303.59(Max Conc. mM)
Ethanol:100.0(Max Conc. mg/mL);303.59(Max Conc. mM)
Water:0.67(Max Conc. mg/mL);2.03(Max Conc. mM) | [form ]
Powder | [pka]
4.72±0.10(Predicted) | [color ]
Light yellow to brown | [InChI]
InChI=1S/C16H27NO6/c1-11(2)14(20)22-12(3)23-15(21)17-10-16(9-13(18)19)7-5-4-6-8-16/h11-12H,4-10H2,1-3H3,(H,17,21)(H,18,19) | [InChIKey]
TZDUHAJSIBHXDL-UHFFFAOYSA-N | [SMILES]
C1(CNC(OC(OC(=O)C(C)C)C)=O)(CC(O)=O)CCCCC1 | [CAS DataBase Reference]
478296-72-9(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
Gabapentin enacarbil (GEn) is an actively transported prodrug of gabapentin that provides sustained doseproportional exposure to gabapentin and predictable bioavailability. In April 2011, Gabapentin enacarbil is approved by the US Food and Drug Administration for the treatment of moderate-to-severe primary restless legssyndrome (RLS) in adults.
Gabapentin enacarbil was designed to be recognized as a substrate for two high-capacity nutrient transports, monocarboxylate transporter type 1 and sodium-dependent multivitamin transporter, and to be efficiently cleaved after absorption to give gabapentin. The separated enantiomers of gabapentin enacarbil have similar cleavage rates in human tissues. Preclinical studies showed that gabapentin enacarbil provides good systemic exposure of gabapentin in rats and monkeys. | [Originator]
Xenoport (United States) | [Uses]
Gabapentin enacarbil (HORIZANT GlaxoSmithKline/XenoPort) is a prodrug of gabapentin used as an anticonvulsant as well as a treatment for neurogenic pain, with the same mechanism of action as pregabalin. | [Definition]
ChEBI: A carbamate ester that is the N-[1-(isobutyryloxy)ethoxy]carbonyl derivative of [1-(aminomethyl)cyclohexyl]acetic acid. The prodrug for gabapentin, used for treatment of neuropathic pain and restless legs syndrome. | [Preparation]
Gabapentin enacarbil is prepared as a racemic mixture from gabapentin either by sequential coupling with 1-chloroethyl chloroformate in the presence of trimethylsilyl chloride and triethylamine followed by addition of isobutyric acid or by direct coupling with an activated 1-(isobutyryloxy) ethyl carbonate. | [Brand name]
Horizant | [Pharmacokinetics]
Gabapentin enacarbil is an acyloxyalkylcarbamate prodrug of analgesic and anticonvulsant drug gabapentin which has problematic pharmacokinetic properties, including short half-life, saturable absorption, high inter-patient variability, and lack of linear dose–response relationship. Gabapentin enacarbil was designed to be absorbed throughout the entire length of the gastrointestinal tract, and its absorption is mediated by high-capacity nutrient transporters, including monocarboxylate transporter 1 (MCT-1) and sodium-dependent multivitamin transporter (SMVT). Prodrug modification produced an extended release of gabapentin with twofold improved, more predictable, and dose-proportional oral bioavailability in humans. During and after its absorption, gabapentin enacarbil is efficiently hydrolyzed by nonspecific esterases to yield gabapentin. Currently, gabapentin enacarbil is commercially available for the treatment of restless legs syndrome and post-herpetic neuralgia of adults.
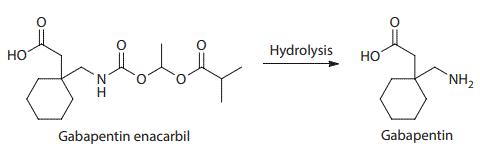
Structure and hydrolysis of gabapentin enacarbil to the active gabapentin | [Clinical Use]
Gabapentin enacarbil is a prodrug of gabapentin (Neurontin,
Pfizer) which binds to the a2-d subunit of L-type voltage-regulated
calcium channels, reducing the release of several neurotransmitters.
122,123 Gabapentin enacarbil was discovered at XenoPort, codeveloped
with GlaxoSmithKline, is marketed under the brand
name Horizant, and is approved for the treatment of moderate
to severe restless leg syndrome. Gabapentin enacarbil was designed
to increase the absorption of gabapentin through the interaction with sodium-dependent multivitamin transporter (SMVT)
and monocarboxylate transporter type 1 (MCT-1). As a result, the
drug demonstrated much better oral bioavailability and more consistent
exposure compared to the parent. | [Synthesis]
Gabapentin 155 was treated with chlorotrimethylsilane and
triethylamine followed by acylation with 1-chloroethyl chloroformate
156 to give acid 157 after hydrolysis of the intermediate silyl
ester. This acid was then used without purification and reacted
with isobutyric acid (158) and triethylamine to afford gabapentin
enacarbil (XIII) in 9.1% overall yield after crystallization. Alternatively,
gabapentin 155 was reacted directly with the fully elaborated
p-nitrophenyl activated side chain 161 in the presence of
potassium carbonate. The resulting mixture of products and
p-nitrophenol was treated with 10% Pd/C and potassium formate
followed by acidic workup to remove the resulting aniline, providing
gabaentin enacarbil (XIII) in 36% overall yield from p-nitrophenol
159 after crystallization. The required activated side chain 161
was prepared from p-nitrophenol 159 via a two-step, one-pot process
involving acylation of the phenol with 1-chloroethyl chloroformate
156 in triethylamine. This provided intermediate 160
which was alkylated with isobutyric acid (158) in the presence of
zinc oxide and potassium iodide, ultimately furnishing the mixed
carbonate 161.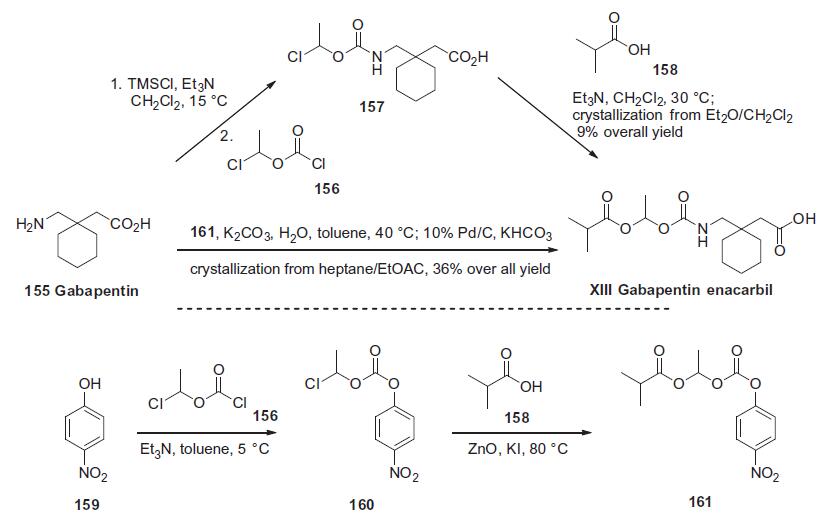
| [storage]
Store at -20°C | [Mode of action]
Gabapentin enacarbil is a prodrug of gabapentin and, accordingly, its therapeutic effects in RLS and PHN are attributable to gabapentin.
The mechanism of action by which gabapentin is efficacious in PHN is unknown but in animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). Gabapentin prevents pain-related responses in several models of neuropathic pain in rats and mice (e.g., spinal nerve ligation models, spinal cord injury model, acute herpes zoster infection model). Gabapentin also decreases pain-related responses after peripheral inflammation (carrageenan footpad test, late phase of formalin test), but does not alter immediate pain-related behaviors (rat tail flick test, formalin footpad acute phase). The relevance of these models to human pain is not known. |
|
|