| Identification | More | [Name]
Hyperoside | [CAS]
482-36-0 | [Synonyms]
HYPERIN
HYPEROSID
HYPEROSIDE
QUERCETIN-3B-D-GALACTOSIDE
QUERCETIN-3-D-GALACTOSIDE
QUERCETIN-3-GALACTOSIDE
QUERCETIN-3-O-GALACTOSIDE
4h-1-benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3-(beta-d-galactopyranosyloxy)-5
hyperasid
hyperozide
2-(3,4-dihydroxyphenyl)-3-(beta-D-galactopyranosyloxy)-5,7-dihydroxy-4H-1-benzopyran-4-one
HYPEROSIDE 98.0% BY HPLC
4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3-(.beta.-D-galactopyranosyloxy)-5,7-dihydroxy-
HYPEROSIDE(P)
QUERCETIN-3-O-BETA-GALACTOSIDE
HYPEROSIDE WITH HPLC
HYPEROSIDE hplc
3-(β-D-galactopyranosyloxy)-5,7-dihydroxy-
Hyperin, Hyperoside
Hyperoside,Hyperin,Quercetin 3-D-galactoside | [EINECS(EC#)]
207-580-6 | [Molecular Formula]
C21H20O12 | [MDL Number]
MFCD00016933 | [Molecular Weight]
464.38 | [MOL File]
482-36-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
225-226°C | [Boiling point ]
872.6±65.0 °C(Predicted) | [density ]
1.87±0.1 g/cm3(Predicted) | [storage temp. ]
-20°C | [solubility ]
DMSO (Slightly), Methanol (Slightly, Heated) | [form ]
Solid | [pka]
6.17±0.40(Predicted) | [color ]
Light Yellow to Yellow | [BRN ]
5784795 | [Major Application]
pharmaceutical small molecule | [InChIKey]
OVSQVDMCBVZWGM-WBVCLXJUNA-N | [SMILES]
O([C@H]1[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O1)O)C1C(C2=C(C=C(O)C=C2OC=1C1C=CC(O)=C(O)C=1)O)=O |&1:1,2,3,5,7,r| | [LogP]
-0.111 (est) | [CAS DataBase Reference]
482-36-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S22:Do not breathe dust .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
DJ3009200
| [F ]
10-23 | [HS Code ]
29389090 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral |
| Questions And Answer | Back Directory | [Abstract]
Hyperoside is an active ingredient of traditional Chinese medicine extracted from Hypericum perforatum. Modern pharmacological studies have shown that it has a strong analgesic effect, and it could protect the heart, brain, liver, anti-myocardial hypoxia damage and protect cerebral ischemic injury. In recent years, the development of hypericin for the treatment of depression, hepatitis B and other diseases has become a hotspot both in domestic and foreign research. | [Extraction]
1. According to Zhongcheng Ke and others, the volume fraction of ethanol, the ratio of material to liquid and the extraction time were independent variables, the extraction rate of hyperoside was the dependent variable, Through the regression of the independent variables and the dependent variables, extraction technology is screened by response surface method predict the best extraction conditions. The results showed that the extraction process was 11.0 times the amount of 77.6% ethanol, and twice with each for 2.7 h.
2. According to Zhuoheng Li and others, taking ethanol concentration, ethanol addition factor, extraction temperature and extraction times as the influencing factors, using the extraction rate of hyperoside as an index, the orthogonal experiment was used to optimize the optimum conditions of hyperoside ethanol extraction. The optimum extraction process is 20 times the amount of 60% ethanol, and 4 times at 90 ℃ with each for 2 h. The method is simple, accurate and reproducible. | [Natural occurance]
Hyperoside is an important natural substance extracted from the dried flowers of the mallow leaves. It is a pale yellow needle-like crystal, and soluble in ethanol, methanol, acetone and pyridine, and stable usually. It could react with hydrochloric acid-magnesium powder to generate cherry red material.
Hyperoside is a flavonol glycoside compound. Hyperoside also has a strong role in inhibiting oogidity reductase, and may be beneficial to the prevention of diabetic cataracts. | [Synthetic method]
Hyperoside is a natural extract and can also be synthesized now. For the synthesis process, re-read the relevant literature to add. Rutin was used as raw material to obtain hyperoside by benzylation, acid hydrolysis, glycoside condensation through phase transfer catalyzing, deacetylation and debenzylation.
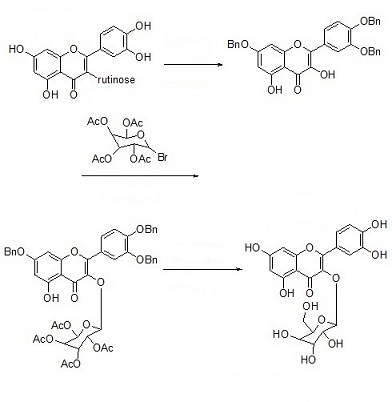 | [Pharmacological effects]
- Pharmacological effects
- Hyperoside has a significant local analgesic effect with no dependence. Its analgesic effect is weaker than morphine, stronger than aspirin, and it is a new type of local analgesic drug.
- The drug has shown a good protective effect on myocardial ischemia and reperfusion, cerebral ischemia and reperfusion, cerebral infarction.
- Hypericin has a significant anti-inflammatory effect: rats implanted wool ball, intraperitoneal injection for 7 days with 20mg/Kg daily, significantly inhibiting the inflammatory process.
- The drug has a strong cough effect.
- Hyperoside also has a strong role in inhibiting oogidity reductase, and may be beneficial to the prevention of diabetic cataracts.
|
| Hazard Information | Back Directory | [Description]
Hypericin, which is widely found in various plants, such as Hypericum, Rosaceae,
Campanulaceae, Labiatae, Rhododendron, Asteraceae Kwai, Garciniaceae,
Leguminosae, Euonymus, and other fruits and whole grass, is a flavonoid compound. | [Chemical Properties]
Yellow Solid | [Physical properties]
Appearance: yellowish needlelike crystals. Melting point: 227–229 °C. Specific
optical rotation: ?83° (c = 0.2, pyridine). Solubility: soluble in ethanol, methanol,
acetone, and pyridine. Hydrochloric acid–magnesium powder reaction yielded formation
of cherry red; ferric chloride reaction was green; α-naphthol reaction was
positive. | [History]
In 1960, Nair et al. isolated hyperin from redosier dogwood, in which its content
is 0.075%. Flavonoids in the treatment of cardiovascular and cerebrovascular diseases
play a pivotal role, among which rutin is a typical representative. Isoquetin mainly presents in the leaves of kumquat flowers and the oleander plant kenaf, and it can also be obtained by chemical synthesis. It shows an anti-inflammatory effect through the capillary permeability test and other animal experiments. It also has a toxic effect on the larvae of Chilohabala larvae. It is one of the active ingredients of Hypericum japonicum, which is used in the treatment of hepatitis because of its inhibition of the activities of hepatic enzyme. | [Uses]
A major flavonoid in apple peels; a bioactive constituent of apple peels | [Definition]
ChEBI: A quercetin O-glycoside that is quercetin with a beta-D-galactosyl residue attached at position 3. Isolated from Artemisia capillaris, it exhibits hepatoprotective activity. | [Indications]
This product is available in the British Pharmacopoeia (2017) and the European
Pharmacopoeia (9.0th ed.).
Monomeric compounds are currently used clinically. In clinical practice, hypericin
is the main active ingredient of proprietary Chinese medicines, such as
Acanthopanax capsules, of which Acanthopanax stem and leaf extract are the raw
materials for the preparation. Xin’an capsules, prepared with hawthorn leaf extract,
are rich in flavonoids, in which hyperoside, a kind of flavonoid, is one of the main
components. Qi yue lipid-lowering tablets are prepared from the effective parts of
Chinese medicinal herbs such as hawthorn (Nucleation) and Astragalus membranaceus.
Flavonoid is one of the main active ingredients in hawthorn, in which hyperoside
content is higher. Xinxuening tablets, containing ursolic acid, vitexin
rhamnoside, hyperoside, citric acid, and others, is a preparation made from hawthorn
and pueraria and other traditional Chinese medicines, in which hawthorn
enhances the actions of the medicine. Clinical indications of Xinxuening tablets are
coronary heart disease, angina pectoris, chest tightness, palpitations, high blood
pressure, arrhythmia, hyperlipidemia, and mental depression. | [General Description]
Produced and qualified by HWI pharma services GmbH.
Exact content by quantitative NMR can be found on the certificate. | [Biochem/physiol Actions]
Protects against peroxide-induced oxidative damage to cells by scavenging reactive oxygen species and enhancing activity of anti-oxidant enzymes, in particular, catalase and glutathione peroxidase. | [Pharmacology]
Hyperoside has a protective effect against myocardial ischemia. It achieves its protective effect on the myocardium by reducing the apoptosis rate of myocardial cells,
inhibiting myocardial cell lactate dehydrogenase release, enhancing anti-freeradical
effect , and inhibiting calcium influx so as to .
Hyperoside has a protective effect against cerebral ischemia. Hyperoside can
significantly lower oxygen-free radicals, reduce the content of malondialdehyde
and NO in brain tissue, inhibit the decrease of the activity of LDH, SOD and glutathione
peroxidase, and hence result in reduced brain energy, and improve the antianoxic
ability . At the same time, hyperoside can reduce the degree of cerebral edema in ischemia-reperfusion rats .
Hypericin has a significant local analgesic effect that is weaker than that of morphine
and stronger than that of aspirin and does not create dependency; it is a new
type of local analgesic. Studies have shown that the analgesic effect of hyperoside
occurs by reducing the Ca2 + of the pain nerve, thereby inhibiting high potassium-induced
Ca2 + influx, which distinguishes it from morphine and aspirin .
Hyperoside has a significant anti-inflammatory effect and a strong cough effect and inhibits the role of eye aldose reductase, which may be beneficial for the prevention
of diabetic cataracts. Hypericin has an obvious protective effect on liver tissue and gastric mucosa , and its mechanism is related to antioxidant activity and the promotion of a normal return of NO level and improvement of SOD activity.
Hyperoside significantly enhances immune function. In vivo, hyperoside has a significant inhibitory effect on spleen B, T lymphocyte proliferation and peritoneal macrophage phagocytosis, and mouse thymus index; in vitro hyperoside, at a concentration
of 6.25–100 μg/mL, significantly enhanced B, T lymphocyte proliferation and promoted the production of interleukin 2 in T lymphocytes .
In addition, hypericin still has hypolipidemic and antidepressant pharmacological
effects. Its derivative rutin has anti-inflammatory and antiviral effects. Another
derivative, quercetin, is effective at inhibiting expectorant, cough, and asthma for
use in the treatment of chronic bronchitis, coronary heart disease, and hypertension. |
|
|