| Identification | Back Directory | [Name]
Thioridazine | [CAS]
50-52-2 | [Synonyms]
tp21
TP-21
Meleril
Sonapax
Thioril
Mellaril
Melleril
Mallorol
Malloryl
Mellarit
Mellerets
Mellerette
Mellaril-S
Thioridazin
Melleretten
THIORIDAZINE
dl-Thioridazine
melleril(liquid)
THIORIDAZINE BASE
Thioridazine (200 mg)
Thioridazine solution
thioridazineprolongatum
Thioridazine, prolongatum
10-(2-(1-Methylpiperidin-2-yl)
THIORIDAZINE,1.0MG/MLINMETHANOL
10-[2-(1-Methyl-2-piperidyl)]-2-[methylthio] phenothiazine
10-((1-methyl-2-piperidyl)ethyl)-2-(methylthio)-phenothiazin
10-[2-(1-ethyl-2-piperidyl)ethyl]-2-(methylthio)phenothiazine
10-(2-(1-methyl-2-piperidyl)ethyl)-2-(methylthio)-phenothiazin
10-(2-(1-Methyl-2-piperidyl)ethyl)-2-(methylthio)phenothiazine
10-[2-(1-Methyl-2-piperdiyl)ethyl]-2-(methylthio)phenothiazine
2-methylmercapto-10-(2-n-methyl-2-piperidyl)ethyl)phenothiazine
Phenothiazine, 10-((1-methyl-2-piperidyl)ethyl)-2-(methylthio)-
3-methylmercapto-n-[2’-(n-methyl-2-piperidyl)ethyl]phenothiazine
2-Methylmercapto-10-(2-(N-methyl-2-piperidyl)ethyl)phenothiazine
Phenothiazine, 10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylthio)-
10-[2-(1-methyl-2-piperidyl)ethyl]-2-methylthio-10h-phenothiazine
10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanylphenothiazine
10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanyl-phenothiazine
10-(2-(1-methyl-2-piperidinyl)ethyl)-2-(methylthio)-10h-phenothiazin
10-(2-(1-Methylpiperidin-2-yl)ethyl)-2-(methylthio)-10H-phenothiazine
10H-Phenothiazine, 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)-
10-[2-(1-Methyl-2-piperidinyl)ethyl]-2-(methylsulfanyl)-10H-phenothiazine
Phenothiazine, 10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylthio)- (6CI, 8CI)
10H-Phenothiazine, 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)- (9CI) | [EINECS(EC#)]
200-044-2 | [Molecular Formula]
C21H26N2S2 | [MDL Number]
MFCD00242875 | [MOL File]
50-52-2.mol | [Molecular Weight]
370.57 |
| Chemical Properties | Back Directory | [Appearance]
Colorless crystals. Soluble in
water and alcohol. | [Melting point ]
72-74° | [Boiling point ]
bp0.02 230° | [density ]
1.1693 (rough estimate) | [refractive index ]
1.5800 (estimate) | [Fp ]
9℃ | [storage temp. ]
?20°C | [solubility ]
Practically insoluble in water, very soluble in methylene chloride, freely soluble in methanol, soluble in ethanol (96 per cent). | [form ]
neat | [pka]
9.5(at 25℃) | [color ]
Colorless to off-white | [Water Solubility ]
1.113mg/L(22.5 ºC) | [InChI]
InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | [InChIKey]
KLBQZWRITKRQQV-UHFFFAOYSA-N | [SMILES]
C1=C2C(SC3=C(N2CCC2CCCCN2C)C=CC=C3)=CC=C1SC | [NIST Chemistry Reference]
10H-Phenothiazine, 10-[2-(1-methyl-2-piperidyl)ethyl]-2-methylthio-(50-52-2) |
| Hazard Information | Back Directory | [Definition]
ChEBI: A phenothiazine derivative having a methylsulfanyl subsitituent at the 2-position and a (1-methylpiperidin-2-yl)ethyl] group at the N-10 position. | [Description]
An antipsychotic drug of the phenothiazine class. It
is of particular interest because of its “atypical” properties,
some of which may be due to its extensive bioconversion to
active metabolites. The therapeutic and side effects of thioridazine and its metabolites involve blockade of brain dopamine
receptors, but also actions mediated via blockage of muscarininc
cholinergic and α-adrenergic receptors. | [Chemical Properties]
Colorless crystals. Soluble in
water and alcohol. | [Originator]
Mellaril,Sandoz,US,1959 | [Uses]
In terms of antipsychotic activity, thioridazine is inferior to aminazine. It is most effec�tive in mental and emotional disorders accompanied by fear, stress, and excitement. It is
prescribed for various forms of schizophrenia, psychosis, and neurosis. | [Uses]
Mellaril(Novartis). | [Manufacturing Process]
N-(m-methylmercapto-phenyl)-aniline (MP 59° to 61°C) is prepared by
condensing m-methylmercapto-aniline (BP 163° to 165°C/16 mm Hg) with the
potassium salt of o-chloro-benzoic acid and decarboxylating the resultant N-
(m-methylmercapto-phenyl)-anthranilic acid (MP 139° to 141°C) by heating,
and then distilling.
9.87 parts of N-(m-methylmercapto-phenyl)-aniline are heated with 2.93
parts of sulfur and 0.15 part of powdered iodine for 15 minutes in a bath at
about 160°C. Upon termination of the ensuing evolution of hydrogen sulfide,
animal charcoal is added to the reaction mixture and recrystallization carried
out first from 40 parts by volume of chlorobenzene, and then from 25 to 30
parts by volume of benzene at the boiling temperature. The obtained citronyellow
3-methylmercapto-phenothiazine has a MP of 138° to 140°C.
17.82 parts of 2-methylmercapto-phenothiazine, 3.4 parts of finely pulverized sodamide and 80 parts by volume of absolute xylene are heated to boiling for
two hours at a bath temperature of 180°C under a reflux condenser and while
stirring the reaction mixture. Without interrupting the heating, a solution of
13.2 parts of 2-(N-methyl-piperidyl-2')-1chloro-ethane in 40 parts by volume
of absolute xylene is then added dropwise in the course of 1 1/2 hours. After
further heating for 3 hours, the reaction mixture is cooled and, after the
addition of 5 parts of ammonium chloride, is shaken three times with water,
using 25 parts by volume each time. The xylene solution is extracted once
with 35 parts by volume of 3 normal acetic acid and then three times, each
time with 15 parts by volume of the said acid, after which the acetic acid
extract is washed with 60 parts by volume of ether and is then made
phenolphthalein-alkaline by means of 25 parts by volume of concentrated
aqueous caustic soda solution.
The precipitated oily base is taken up in a total of 100 parts by volume of
benzene. The benzene layer, dried over potassium carbonate, is filtered and
then evaporated under reduced pressure. The residue from the evaporation is
distilled in a high vacuum; after separating a preliminary distillate which
passes over up to 228°C under a pressure of 0.92 mm Hg, the principal
fraction, 2-methylmercapto-10-[2'-(N-methyl-piperidyl-2'')-ethyl-
1']phenothiazine, which distills over at 228° to 232°C under the lastmentioned
pressure, is collected. The analytically pure base has a BP of
230°C/0.02 mm Hg. | [Therapeutic Function]
Tranquilizer | [Synthesis]
Thioridazine, 10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylthio)phenoth�iazine (6.1.9), is synthesized in an analogous manner by alkylating 2-methylthiophenoth�iazine with 2-(2-chloroethyl)-1-methylpiperidine [29,30].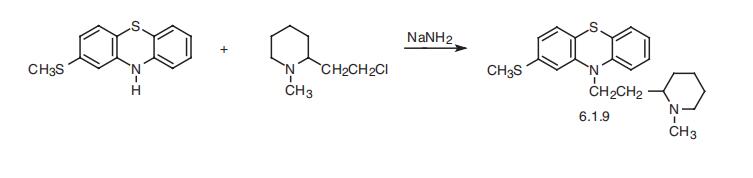
|
|
| Company Name: |
parabiochem
|
| Tel: |
025-83453382-8005 |
| Website: |
www.parabiochem.cn |
|