| Identification | Back Directory | [Name]
Praziquantel | [CAS]
55268-74-1 | [Synonyms]
Cesol
Azinox
WarMnil
droncit
pyquiton
Prazinon
Cysticide
embay8440
Distocide
biltricide
praz,quantel
PRAZIQUANTEL
Praziquentel
Praziguantel
Prazichantel
Usnea diffract
PraziquantelUsp25
PRAZIQUANTEL BP 98
Praziquintel (FDA)
Praziquantel (200 mg)
Praziquantel, Pyquiton
PRAZIQUANTEL F&D VERSION
Praziquantel (Biltricide)
PRAZIQUANTEL VETRANAL, 250 MG
Praziquantel for system suitability
2-cyclohexylcarbonyl-1,2,3,6,7,11b-hexahydro-4h-pyrazino(2,1-a)isoquinolin-4
4h-pyrazino(2,1-a)isoquinolin-4-one,2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexah
2-[CYCLOHEXYLCARBONYL]-1,2,3,6,7-11B-HEXAHYDRO-4H-PYRAZINO[2,1A]ISOQUINOLIN-4-ONE
2-cyclohexyl-carbonyl-1,3,4,6,7,11b-hexahydro-2h-pyrazine(2,1-a)isoquinoline-4-one
2-(Cyclohexanecarbonyl)-1,3,4,6,7,11b-hexahydro-4H-pyrazino[2,1-α]isoquinolin-4-one
2-(cyclohexanecarbonyl)-2,3,6,7-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one
1-a]-isoquinolin-4-one,2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino-[2
4H-pyrazino-[2,1-a]-isoquinolin-4-one,2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-
2-(CYCLOHEXYLCARBONYL)-1,2,3,6,7-11BETA-HEXAHYDRO-4H-PYRAZINO[2,1A] ISOQUINOLIN-4-ONE
(11bS)-2-cyclohexanecarbonyl-1H,2H,3H,4H,6H,7H,11bH-piperazino[2,1-a]isoquinolin-4-one
4H-Pyrazino[2,1-a]isoquinolin-4-one, 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-(9CI) | [EINECS(EC#)]
259-559-6 | [Molecular Formula]
C19H24N2O2 | [MDL Number]
MFCD00058531 | [MOL File]
55268-74-1.mol | [Molecular Weight]
312.41 |
| Chemical Properties | Back Directory | [Melting point ]
136-138 C | [Boiling point ]
1377℃ | [density ]
1.1209 (rough estimate) | [refractive index ]
1.5600 (estimate) | [Fp ]
>110°(230°F) | [storage temp. ]
−20°C
| [solubility ]
ethanol: soluble5mg/mL | [form ]
powder or crystals | [pka]
-0.98±0.20(Predicted) | [color ]
Crystals from EtOAc/hexane | [Water Solubility ]
Freely soluble in ethanol or dichloromethane. Slightly soluble in water
| [Merck ]
13,7802 | [BRN ]
761557 | [BCS Class]
2 | [Major Application]
clinical testing | [InChI]
1S/C19H24N2O2/c22-18-13-20(19(23)15-7-2-1-3-8-15)12-17-16-9-5-4-6-14(16)10-11-21(17)18/h4-6,9,15,17H,1-3,7-8,10-13H2 | [InChIKey]
FSVJFNAIGNNGKK-UHFFFAOYSA-N | [SMILES]
O=C1CN(CC2N1CCc3ccccc23)C(=O)C4CCCCC4 | [EPA Substance Registry System]
4H-Pyrazino[2,1-a]isoquinolin-4-one, 2-(cyclohexylcarbonyl)-1,2, 3,6,7,11b-hexahydro-(55268-74-1) |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Usage]
Anthelmintic, effective against flatworms. | [Usage]
anthelmintic; EMBAY-8440 | [Description]
Praziquantel (PZQ) is an isoquinoline derivative with most of the biological activity found in the
levo enantiomer. The compound has no activity against nematodes, but it is highly effective
against cestodes and trematodes. | [Originator]
Cesol ,Merck ,W. Germany ,1980 | [Definition]
ChEBI: 2-[cyclohexyl(oxo)methyl]-3,6,7,11b-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4-one is a member of isoquinolines. | [Manufacturing Process]
15 g of a nickel-aluminum alloy (1:1) is introduced in incremental portions and under agitation into 200 ml of 20% sodium hydroxide solution within 5 minutes; the mixture is maintained at 80°C for 45 minutes, then allowed to settle, decanted off, washed with water, and 1,000 ml of 1% (-)-tartaric acid solution is added thereto, adjusted to pH 5 with 1 N sodium hydroxide solution. The mixture is heated under agitation for 90 minutes to 80°C, decanted, and washed with water and methanol. The thus-obtained (-)tartaric acid-Raney nickel catalyst is added to a solution of 2cyclohexylcarbonyl-4-oxo-2,3,6,7-tetrahydro-4H-pyrazino[2,1-a]isoquinoline. The reaction mixture is hydrogenated under normal pressure and at room temperature. After the catalyst has been filtered off and the solvent evaporated, 2-cyclohexylcarbonyl-4-oxo-1,2,3,6,7,11b-hexahydro-4Hpyrazino[2,1-a]isoquinoline, melting point 136°C to 138°C, is produced. | [Brand name]
Biltricide (Bayer). | [Therapeutic Function]
Anthelmintic | [Acquired resistance]
There is evidence that resistance to praziquantel is emerging
in schistosomes, although there is debate as to whether treatment
failures are due to resistance or innate tolerance. | [Pharmaceutical Applications]
A synthetic pyrazinoquinoline formulated for oral administration.
It is stable in the dry state, but hygroscopic. | [Mechanism of action]
Praziquantel is readily absorbed (80% in 24 hours)
after oral administration, with serum concentrations being
maximal in 1 to 3 hours; the drug has a half-life of
0.8 to 1.5 hours. Its bioavailability is reduced by phenytoin
or carbamazepine and increased by cimetidine.
Dexamethasone decreases plasma praziquantel levels
by 50%. Praziquantel is excreted by the kidneys. | [Pharmacokinetics]
Oral absorption: >80%
Cmax 50 mg/kg oral: 1 mg/L after 1–2 h
Plasma half-life: parent drug: 1–1.5 h
metabolites: 4–6h
Plasma protein binding: 80%
Praziquantel is rapidly absorbed when given orally, but it
undergoes extensive first-pass biotransformation and the concentration
of unchanged drug in plasma is low. The major
metabolite, a 4-hydroxy derivative, retains little to no antiparasitic
activity. About 80% of the oral dose, as parent drug
and its metabolites, is excreted in the urine by the fourth day
post-treatment, 90% of this in 24 h. A higher peak plasma
concentration is achieved in infected people, but other pharmacokinetic
values are unchanged. | [Clinical Use]
2-(Cyclohexylcarbonyl)-1,2,3,6,7, 11b-hexahydro-4Hpyrazino[2,1-a]isoquinolin-4-one (Biltricide) is a broadspectrumagent that is effective against various trematodes (flukes). It has become the agent of choice for the treatmentof infections caused by schistosomes (blood flukes).
The drug also provides effective treatment for fasciolopsiasis(intestinal fluke), clonorchiasis (Chinese liver fluke),fascioliasis (sheep liver fluke), opisthorchosis (liver fluke),and paragonimiasis (lung fluke). Praziquantel increases cellmembrane permeability of susceptible worms, resulting inthe loss of extracellular calcium. Massive contractions andultimate paralysis of the fluke musculature occurs, followedby phagocytosis of the parasite.
Following oral administration, about 80% of the doseis absorbed. Maximal plasma concentrations are achievedin 1 to 3 hours. The drug is rapidly metabolized in theliver in the first-pass. It is likely that some of the metabolitesare also active. Praziquantel occurs as a white crystallinesolid that is insoluble in water. It is available as600-mg film-coated tablets. The drug is generally welltolerated. | [Clinical Use]
Praziquantel is an extremely active broad-spectrum
anthelmintic that is well tolerated. It is the most effective
of the drugs used in the treatment of schistosomiasis,
possessing activity against male and female adults
and immature stages. Unlike other agents, it is active
against all three major species (S. haematobium, S. mansoni,
and S. japonicum). In addition, it has activity
against other flukes, such as C. sinensis, Paragonimus
westermani, O. viverrini, and the tapeworms (D. latum,
H. nana, T. saginata, and T. solium). It is not as effective
against F. hepatica. It is used effectively in the treatment
of clonorchiasis and paragonimiasis and is an effective
alternative agent to niclosamide in the treatment of
tapeworm infestations. | [Clinical Use]
Schistosomiasis
Other trematode infections (except F. hepatica)
Tapeworm infection, including cerebral cysticercosis
Treatment may need to be prolonged in cerebral cysticercosis. | [Safety Profile]
Poison by intraperitoneal route.Moderately toxic by ingestion and other routes. Humanmutation data reported. When heated to decomposition itemits toxic fumes of NOx. | [Synthesis]
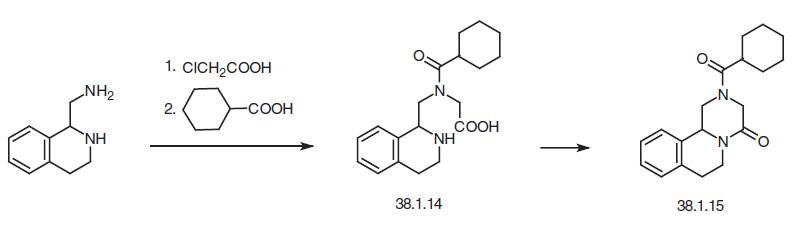
Praziquantel, 2-(cyclcohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino [2,1a]isoquinolin-4-one (38.1.15), is a derivative of pyrazinoquinoline that is made in two ways. According to one of them, 1-aminomethyl-1,2,3,4-tetrahydroiso�quinoline is alkylated with chloroacetic acid, and then the resulting amine is acylated with cyclohexanecarbonyl chloride to make 1-(N-carboxymethyl-N-cyclohexylcarbonyl�aminomethyl)-1,2,3,4-tetra-hydroisoquinoline (38.1.14), which is heated at 150°C to give the desired praziquantel.
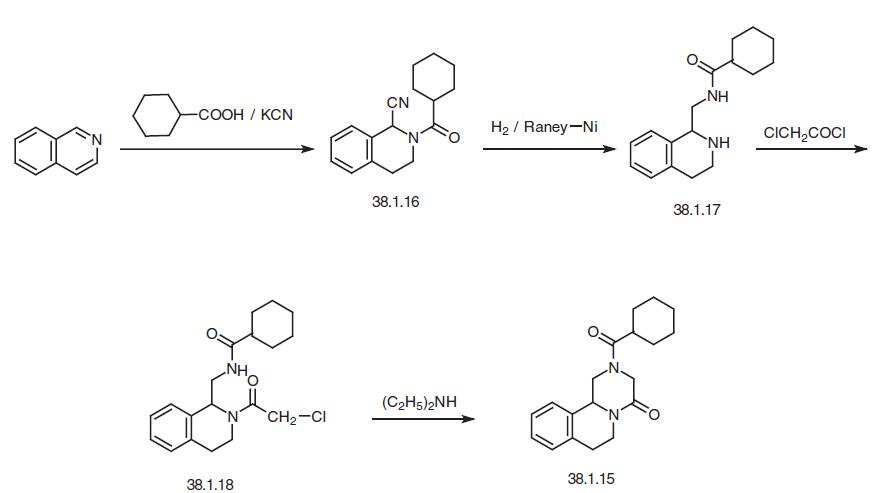
Another way of synthesizing this drug begins with isoquinoline, which is reacted with a mixture of cyclohexanecarbonyl chloride / potassium cyanide to make a dihydro derivative of isoquinoline (38.1.16). This is reduced by hydrogen over Raney nickel to give the reduction–reamidation product—the amide 1-(N-cyclohexylcarbonylaminomethyl)- 1,2,3,4-tetrahydroisoquinoline (38.1.17). Acylating this with chloracetic acid chloride gives a chlroacetyl derivative (38.1.18), which when heated in the presence of diethylamine results in an intramolecular alkylation, giving the desired product—prazi quantel.
| [Veterinary Drugs and Treatments]
Praziquantel is indicated for (approved labeling) for the treatment
of Dipylidium caninum, Taenia pisiformis, and Echinococcus granulosis
in dogs, and Dipylidium caninum and Taenia taeniaeformis in
cats. Fasting is not required nor recommended before dosing. A
single dose is usually effective, but measures should be taken to prevent
reinfection, particularly against D. caninum. Praziquantel can
also be used for treating Alaria spp. in dogs and cats and Spirometra
mansonoides infections in cats.
Praziquantel has been used in birds and other animals, but it
is usually not economically feasible to use in large animals. In humans,
praziquantel is used for schistosomiasis, other trematodes
(lung, liver, intestinal flukes) and tapeworms. It is not routinely effective
in treating F. hepatica infections in humans.
Combination products can give a wide spectrum of internal
parasite control in a variety of species. | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Antiepileptics: concentration reduced by
carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antimalarials: concentration reduced by chloroquine.
Ulcer-healing drugs: concentration reduced by
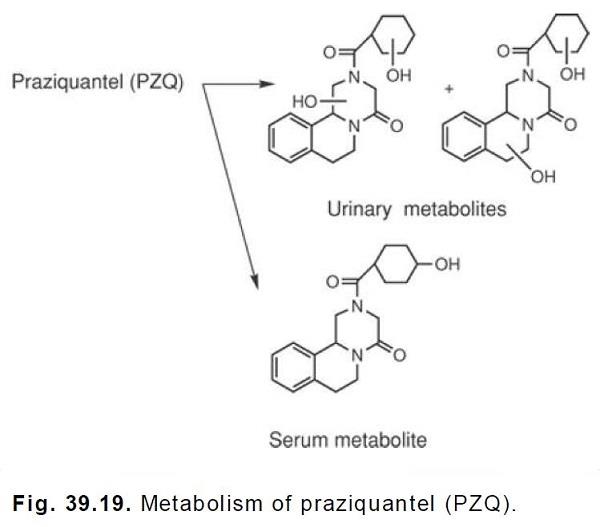
cimetidine. | [Metabolism]
Praziquantel is rapidly absorbed and undergoes hepatic first-pass metabolism. The metabolites are
either less active or inactive and consist of hydroxylated compounds. In the serum, the major
metabolite appears to be the monohydroxylated 4-hydroxycyclohexylcarboxylate, whereas in the
urine, 50 to 60% of the initial PZQ exists as dihydroxylated products.These
hydroxylation reactions are catalyzed by CYP2B6 and CYP3A4. The metabolites would be
expected to exist in the conjugated form in the urine.
 |
| Safety Data | Back Directory | [Hazard Codes ]
F,C | [Risk Statements ]
11-34 | [Safety Statements ]
16-26-36/37/39-45 | [WGK Germany ]
1
| [RTECS ]
UQ4150000
| [HS Code ]
29142900 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Aquatic Chronic 3 | [Toxicity]
LD50 in mice, rats (mg/kg): 2000-3000 orally; >3000 s.c. (Muermann) |
| Questions And Answer | Back Directory | [Pharmacology and mechanism of action]
Praziquantel is a pyrazinoquinoline compound originally developed for the treatment of schistosomiasis but has been found to have a wide spectrum of anthelminthic activity. Praziquantel is a racemate but the R (+) enantiomer is solely responsible for its antiparasitic activity. It is active against trematodes (all Schistosoma species pathogenic to man, Paragonimus westermani, and Clonorchis sinensis) and cestodes (Taenia saginata, Taenia solium, Hymenolepis nana and Diphyllobothrium latum) 【1】. The mechanism of action of praziquantel is not clearly known. Schistosomes take up the drug rapidly. Drug uptake is immediately followed by increased muscular activity that proceeds to tetanic contraction and vacuolization of the parasite tegument 【2】. The muscular effects of the drug are presumed to be responsible for the shift of the parasites from the mesenteric veins to the liver in vivo. However, hepatic shift has been demonstrated with most known schistosomicides and may not provide any specific information of the drug’s mechanism of action. Recent experimental findings have suggested that the antischistosomal effects of the drug are related to its effect on the tegument rather than on the musculature 【3】. Another pharmacological effect of the drug includes an increase of membrane permeability to cations, particularly calcium 【4】. However, the role of this effect to the anthelminthic property of the drug is unknown.
| [Indications]
Infections caused by Schistosoma species pathogenic to man (Schistosoma haematobium, S. mansoni, S. japonicum and S. mekongi). The drug is most cost-effective in mixed infections. It is also effective for infections with flukes (Paragonimus westermani and Clonorchis sinensis) and in cestodes (Hymenolepis nana, Diphyllobotrium latum, Taenia saginata, T. solium) including the larval stage of Taenia solium (cysticercosis).
Praziquantel has some effect against fascioliasis, but triclabendazole, a new anthelminthic drug still under clinical evaluation is more effective.
| [Side effects]
In large-scale and community-based studies in patients and healthy volunteers, the drug showed only mild to moderate and transient side effects 【5—11】. The frequency and intensity of side effects seemed to be dose related. In one study【10】, the frequency of the side effects were: dizziness (29%), headache (15%), lassitude (19%), pain in the limbs (22%), and abdominal distress (9%). Nausea, insomnia, fever, and non-itching macular eruptions occurred in single patients. 40% of the patients remained free from any side effects. Abdominal colic and bloody diarrhoea due to praziquantel have been reported by others 【12, 13】. Praziquantel has not shown to be mutagenic or carcinogenic 【14, 15, 16】.
| [Interactions]
Phenytoin, carbamazepine, and dexamethasone have been reported to decrease the plasma concentrations of praziquantel by 10% to 50% 【17, 18】. The clinical relevance of these interactions for the treatment of parasitic infections needs further investigation.
| [Preparations]
Biltricide® (Bayer). Tablets 600 mg.
Cysticide® (E.Merck). Tablets 500 mg.
Cesol® (E.Merck). Tablets 150 mg.
| [The treatment of schistosomiasis]
Schistosomiasis is parasitic disease with both human and animal being prone to get infected. Schistosome has a relative complicated life history. Adult parasites live in the mesenteric vein and portal vein blood of people, cattle, pigs and some other mammals, and therefore humans and these animals are called as the adult host or definitive host.
Praziquantel is a kind of common drugs for treatment of schistosomiasis with an extremely small animal toxicity. After its oral administration, it is rapidly absorbed in the digestive tract. The time of the plasma concentration for reaching peaks: 5 minutes for mice, l5~30 minutes for rat, 30 to 120 minutes for dogs, and 2 h for sheep. After its absorption this drug is widely distributed in all tissues and organs; it is even able to penetrate through the blood-brain barrier of rats and can also enter into the bile of dogs. It can induce of influx of the Ca 2+ located outside of the schistosome parasite muscle cell membrane, and thus causing muscle contractures and loss of ability of sucking parasite location. At the same time, it also causes deficiency in sugar metabolism and energy metabolism, disrupting the "With immunization" state and then working together with the host immune system for finally eliminating the parasites. Therefore, it has good killing efficacy in treating China branch schistosomiasis, tapeworm, lung fluke, cysticercosis and also immature parasites (cercariae and miracidia).
The common side effects of praziquantel are as follows:
1. during the first 1 hour of medication of the first time: dizziness, headache, nausea, abdominal pain, diarrhea, fatigue, aching limbs can occur, usually at a lesser extent and short duration, and does not affect the treatment without specific treatment.
2. in a few cases, there may be symptoms such as heart palpitations, chest tightness, and T wave change and primary contraction in ECG; supraventricular tachycardia and atrial fibrillation can sometimes also happen.
3. in a few cases there may be a transient increase in transaminases and toxic hepatitis.
4. it sometimes can induce mental disorders and gastrointestinal bleeding.
5. hernia, allergic reactions (rash, asthma), etc. are also seen.
| [Chemical Properties]
It is white or almost white crystalline powder; it is odorless with a slightly bitter taste. It also has hygroscopic effect. Solubility (g/100m1): 9.7 in ethanol, 56.7 in chloroform, and 0.04 in water. It is easily soluble in dimethyl sulfoxide (DMSO), but insoluble in ether. It has a melting point of 136~141 ℃. Acute toxicity LD50 in mice and rats (mg/kg): 2000~3000 oral administration,> 3,000 subcutaneously injection.
| [Uses]
It is a kind of broad-spectrum anti-parasitic disease drug. It can be used for the treatment and prevention of schistosomiasis, cysticercosis, paragonimiasis, hydatid disease, fasciolopsiasis, hydatid disease, and worm infection.
It can also be used as anthelmintic and is effective in treating animal gastrointestinal nematodes. It can be mixed in the feed for application.
The product is a kind of anthelmintic drug effective in treating Schistosoma japonicum, Schistosoma mansoni and Schistosoma haematobium, Clonorchis sinensis, Paragonimus westermani, fasciolopsis buski, tapeworms and cysticercosis. It has a especially strong killing effect on tapeworm and is currently of highest efficiency among anti-schistosomiasis drug.
It is a kind of anthelmintics drug mainly used for treating schistosomiasis. It can also used for treating Fahrenheit schistosomiasis, taeniasis, paragonimiasis, and cysticercosis
| [Production method]
There are a variety of synthetic routes (isoquinoline route, piperazine route, and phenethylamine route). Isoquinoline has advantages such as wide sources of initial raw material and low cost. Isoquinoline can be converted to 1-benzoyl-2-cyano-1,2-dihydro-isoquinoline through Reissetr reaction. It is further converted to 1-benzoyl-aminomethyl-1, 2, 3, 4-tetrahydroisoquinoline through pressurized hydrogenation. Then followed by cyclization with chloroacetyl chloride to give 2-benzoyl-1,3, 4,6,7,11b-hexahydro-2H-pyrazino[2,1-b]isoquinolin-4-ketone. Finally, apply phosphoric acid hydrolysis and perform condensation reaction with cyclohexanecarboxylic acid chloride to obtain the final product.
There are a variety of synthetic routes for industrial production including isoquinoline route, piperazine route, and phenethylamine route, among which the isoquinoline route is the best. In this route, first perform adduct reaction between isoquinoline and benzoyl chloride as well as potassium cyanide, further go through catalytic hydrogenation and rearrangement to generate 1-benzoyl-methyl-amino-1,2,3,4-tetrahydroisoquinoline, and then sequentially go through chlorine acetylation, cyclization, hydrolysis under increased pressure, and cyclohexanone acylation to generate the final product.
Take phenethylamine as the raw material, after acylation through chloroacetyl chloride, further introduce the amino group after adding terephthalamide potassium for amination reaction, then have cyclization reaction in the action of phosphorus oxychloride to give 3,4-dihydroisoquinoline derivative; further go through hydrogenation and hydrolysis to obtain 1-aminomethyl-tetrahydroquinoline; successively use cyclohexane carboxylic acid chloride and chloroacetyl chloride for acylation and finally go through dehydrochlorination and cyclization to obtain praziquantel.
You can alternatively use isoquinoline as raw material; it first go through Reissert reaction to introduce a cyano group in l position and have nitrogen benzoylated, followed by hydrogenation while benzoyl group is transferred to the amino group of the side chain, further introduce a chlorine acetyl group to the amino group on the ring, then successively go through cyclization, hydrolysis, cyclohexanone formylation to obtain praziquantel.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| [Category]
Toxic substances
| [Toxicity grading]
Poisoning
| [Acute toxicity]
Oral rat LD50; 2840 mg/kg; Oral-Mouse LD50: 2454 mg/kg.
| [Flammability and hazardous characteristics]
Combustible; combustion produces toxic fumes of nitrogen oxides.
| [Storage Characteristics]
ventilation, low-temperature, and drying.
| [Extinguishing agent]
Dry powder, foam, sand, carbon dioxide, water spray.
| [References]
1.Andrews P, Thomas H, Pohlke R, Seubert J. Praziquantel (1983). Med Res Rev, 3, 147–200.
2. Xiao SH, Friedman PA, Catto BA, Webster LT Jr (1984). Praziquantel induced vesicle formation in the tegument of male mansoni is calcium dependent. J Parasitol, 70, 177–179.
3. Xiao SH, Catto BA, Webster LT Jr, Melborn H, Becker B (1984). Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. J Infect Dis, 151, 1130–1137.
4. Pax R, Bennett JL, Fetterer R (1978). A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn Schmiedebergs Arch Pharmacol, 304, 309–315.
5. Davis A, Biles JE, Ulrich AM, Dixon H (1981). Tolerance and efficacy of praziquantel in phase IIA and IIB therapeutic trials in Zambian patients. Arzneimittelforschung, 31, 568–574.
6. Davis A, Biles JE, Ulrich AM (1979). Initial experiences in patients with Schistosoma mansoni previously treated with oxamniquine and/or hycanthone: Resistance of Schistosoma mansoni to schistosomicidal agents. Trans R Soc Trop Med Hyg, 76, 652–659.
7. Pugh RNH, Teesdale CH (1983). Single dose oral treatment in urinary schistosomiasis: a double blind trial. BMJ, 286, 429–432.
8. Ishizaki T, Kamo E, Boehme K (1979). Double-blind studies of tolerance to Praziquantel in Japanese patients with Schistosoma japonicum infections. Bull WHO, 57, 787–791.
9. Santos AT, Bias BL, Nosenas JS, Portillo GP, Ortega OM, Hayashi M, Boehme K (1979). Preliminary clinical trials with praziquantel in Schistosoma japonicum infections in the Philippines. Bull WHO, 57, 793–799.
10. Zhejiang Clinical Cooperative Research Group for praziquantel (1980). Clinical evaluation of praziquantel in treatment of schistosomiasis japonica. A report of 181 cases. Chin Med J, 93, 375–384.
11. Katz N, Rocha RS, Chaves A (1979). Preliminary trial with praziquantel in human infections due to Schistosoma mansoni. Bull WHO, 57, 781–785.
12. Watt G, Baldovino P, Castro J, Fernando M, Ranoa C (1986). Bloody diarrhea after praziquantel therapy. Trans R Soc Trop Med Hyg, 80, 345–346.
13. Polderman AM, Gryseels B, Gerold JL, Mpamila K, Manshande JP (1984). Side effects of praziquantel in the treatment of Schistosoma mansoni in Maniema, Zaire. Trans R Soc Trop Med Hyg, 78, 752–754.
14. Frohberg H, Schulze Schenking M (1981). Toxicological profile of praziquantel a new drug against cestode and Schistosoma infections as compared to some other schistosomicides. Arzneimittelforschung, 31, 555–565.
15. Pütter J, Held H (1979). Quantitative studies on the occurrence of praziquantel in milk and plasma of lactating women. Eur J Drug Metab Pharmacokinet, 4, 193–198.
16. Billings PC, Heidelberger C (1982). Effects of praziquantel a new antischistosomicide drug on the mutation and transformation of mamalian cells. Cancer Res, 42, 2692–2696.
17. Bittencourt PRM, Gracia CM, Martins R, Fernandes AG, Diekmann HW, Jung W (1992). Phenytoin and carbamazepine decrease oral bioavailability of praziquantel. Neurology, 42, 492–496.
18. Vazquez M, Jung H, Sotelo J (1987). Plasma levels of praziquantel decrease when dexamethasone is given simultaneously. Neurology, 37, 1561–1562.
|
|
|