| Identification | More | [Name]
Dimethyl carbonate | [CAS]
616-38-6 | [Synonyms]
CARBONIC ACID DIMETHYL ESTER
DIMETHYL CARBONATE
DMC
METHYL CARBONATE
CH3OCOOCH3
Dimethyl ester of carbonic acid
Methyl carbonate ((MeO)2CO)
methylcarbonate((meo)2co)
dimetyl carbonate
DIMETHYL CARBONATE, 99+%, ANHYDROUS
DIMETHYL CARBONATE, REAGENTPLUS, 99%
DimethylCarbonateForSynthesis
DimethylCarbonate(Dmc)
dimethly carbonate
Dimethylcarbonate,99%
Dimethylcarbonat
DIMETHYL CARBONATE pure
Carbonic acid hydrogen methyl ester
Methoxyformic acid
Methoxymethanoic acid | [EINECS(EC#)]
210-478-4 | [Molecular Formula]
C3H6O3 | [MDL Number]
MFCD00008420 | [Molecular Weight]
90.08 | [MOL File]
616-38-6.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless liquid | [Melting point ]
2-4 °C (lit.) | [Boiling point ]
90 °C (lit.) | [density ]
1.069 g/mL at 25 °C(lit.)
| [vapor density ]
3.1 (vs air)
| [vapor pressure ]
18 mm Hg ( 21.1 °C)
| [refractive index ]
n20/D 1.368(lit.)
| [Fp ]
65 °F
| [storage temp. ]
Flammables area | [solubility ]
139g/l | [form ]
Liquid | [color ]
<50(APHA)
| [Odor]
Pleasant | [Stability:]
Stable. Highly flammable. Incompatible with strong oxidizing agents, potassium t-butoxide. | [explosive limit]
4.22-12.87%(V) | [Water Solubility ]
139 g/L | [Sensitive ]
Moisture Sensitive | [Merck ]
14,3241 | [BRN ]
635821 | [Dielectric constant]
3.1699999999999999 | [Cosmetics Ingredients Functions]
SOLVENT
PROPELLANT
FRAGRANCE | [Cosmetic Ingredient Review (CIR)]
Dimethyl carbonate (616-38-6) | [InChI]
1S/C3H6O3/c1-5-3(4)6-2/h1-2H3 | [InChIKey]
IEJIGPNLZYLLBP-UHFFFAOYSA-N | [SMILES]
O=C(OC)OC | [LogP]
0.23-0.354 at 20-25℃ | [Surface tension]
28.08mN/m at 298.15K | [CAS DataBase Reference]
616-38-6(CAS DataBase Reference) | [NIST Chemistry Reference]
Carbonic acid, dimethyl ester(616-38-6) | [EPA Substance Registry System]
616-38-6(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F | [Risk Statements ]
R11:Highly Flammable. | [Safety Statements ]
S9:Keep container in a well-ventilated place .
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 1161 3/PG 2
| [WGK Germany ]
1
| [RTECS ]
FG0450000
| [Autoignition Temperature]
458 °C | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29209090 | [Storage Class]
3 - Flammable liquids | [Hazard Classifications]
Flam. Liq. 2 | [Safety Profile]
Moderately toxic by
intraperitoneal route. Mildly toxic by
ingestion. An irritant. Violent reaction or
ignition on contact with potassium-tertbutoxide. A very dangerous fire hazard
when exposed to heat, open flames (sparks),
or oxiduers. To fight fire, use alcohol foam.
When heated to decomposition it emits
acrid smoke and irritating fumes. | [Hazardous Substances Data]
616-38-6(Hazardous Substances Data) | [Toxicity]
LD50 in rats (g/kg): 13.8 orally; 2.5 dermally; LC50 (4 hr) in rats (mg/l): 140 by inhalation (Ono) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Methanol-->Propylene oxide-->PHOSGENE-->CARBON MONOXIDE-->Methyl chloroformate-->Propylene carbonate-->Ethylene carbonate | [Preparation Products]
Diethyl carbonate-->7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-4-oxoquinoline-3-carboxylic acid-->DIMETHYL METHOXYMALONATE-->1-Methylimidazole-->Ciprofloxacin-->N-Methyl-3-carbomethoxy-4-piperidone hydrochloride-->(S)-(-)-1,2,3,4-Tetrahedro-naphthoic acid-->2-PHENYLMALONAMIDE-->4-DIMETHOXYMETHYL-6-HYDROXYPYRIMIDINE-2-THIOL-->1H-Azepine-3-carboxylicacid,hexahydro-,methylester(9CI)-->1,5-Diphenylcarbazide-->Asulam-->4-Bromo-1,3,5-trimethyl-1H-pyrazole-->(1,3,5-TRIMETHYL-1H-PYRAZOL-4-YL)METHYLAMINE-->1,3,5-Trimethylpyrazole-->1,3,5-Trimethyl-1H-pyrazole-4-carboxylic acid-->1,3,5-Trimethyl-1H-pyrazole-4-carboxaldehyde-->1H-Pyrazole-4-carbonyl chloride, 1,3,5-trimethyl- (9CI)-->3-Methylanisole-->Ethyl methyl carbonate |
| Hazard Information | Back Directory | [General Description]
A clear, colorless liquid with a pleasant odor. Flash point 66°F. Denser than water and slightly soluble in water. Vapors are heavier than air. Used to make other chemicals and as a special purpose solvent. | [Reactivity Profile]
DIMETHYL CARBONATE(616-38-6) reacts with acids to liberate heat along with methanol and carbon dioxide. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction with caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides. | [Air & Water Reactions]
Highly flammable. Slightly soluble in water. | [Hazard]
Flammable, dangerous fire risk. Toxic by
inhalation, strong irritant. | [Health Hazard]
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. | [Fire Hazard]
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. | [Definition]
ChEBI: Dimethyl carbonate is a carbonate ester that is carbonic acid in which both hydrogens are replaced by methyl groups. A flammable, colourless liquid (m.p. 2-4°C, b.p. 90°C) with a characterstic ester-like odour, it is used as a 'green' methylating agent and as a solvent. It has a role as a solvent and a reagent. | [Preparation]
Dimethyl carbonate (DMC) is formed by the reaction of MeOH with phosgene or methyl chloroformate in the presence of a concentrated sodium hydroxide solution in a two-phase reaction in high yields and purity. Other alcohols can also be phosgenated. As DMC is now more easily accessible via the direct oxidative carbonylation of MeOH, phosgenation is losing its attractiveness in this application. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 49, p. 1122, 1984 DOI: 10.1021/jo00180a033
Tetrahedron Letters, 15, p. 803, 1974 | [Flammability and Explosibility]
Highlyflammable | [Purification Methods]
If the reagent has broad intense bands at 3300cm-1 and above (i.e. OH stretching), then it should be purified further. Wash it successively with 10% Na2CO3 solution, saturated CaCl2, H2O, and dry it by shaking mechanically for 1hour with anhydrous CaCl2, and fractionate. [Bowden & Butler J Chem Soc 78 1939, Vogel J Chem Soc 1847 1948, Beilstein 3 IV 3.] | [Toxics Screening Level]
The initial threshold screening level (ITSL) for dimethyl carbonate is 300 μg/m3 based on an annual averaging time. |
| Questions And Answer | Back Directory | [Outline]
Dimethyl carbonate is briefly referred to as DMC. At room temperature, it is a colorless and transparent liquid with a pungent odor with a relative density (D204) of 1.0694, a melting point of 4 °C, boiling point of 90.3 °C, the flash point being 21.7 °C (opening) and being 16.7 °C (closed ) and the refractive index (nd20) being 1.3687. It is flammable and non-toxic and is miserable with almost all organic solvents in any proportion with alcohols, ketones and esters. It is slightly soluble in water. It can be used as the methylating agent. Compared with other methylating reagent such as methyl iodide and dimethyl sulfate, dimethyl carbonate is less toxic, and biodegradable.
The past method of making dimethyl carbonate with phosgene as raw materials is not frequently used. Instead, people now adopt the catalytic oxidative carbonylation of methanol in the presence of oxygen which is more environmentally friendly than the previous method.
Dimethyl carbonate can enable to methylation of aniline, phenol and carboxylic acid. However, many reaction demands high-pressure. DBU can be added during reflux of DMC for catalyzing the methylation of carboxylic acid with dimethyl carbonate.
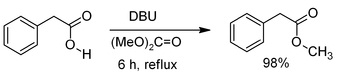
| [Reference quality standards]
Index name battery grade excellent grade first grade qualified experimental methods
Appearance, colorless and transparent liquid
Dimethyl carbonate content% ≥99.9 ≥99.5 ≥99.0 ≥98.5 Gas Chromatography
Water, Water Content% ≤30ppm ≤0.10 ≤0.10 ≤0.10 GB606
Alkalinity mmol/10Og-≤0.10 ≤0.12 ≤0.12 Q/GNPC-JX 017
Non-volatile matter, %-≤0.02 ≤0.02 ≤0.02 GB6324.2
Peroxide (By H2O2) ≤5ppm---GB6016-85
Density (20 ℃), Density g/cm3 1.071 ± 0.005
| [The application of dimethyl carbonate]
1, a novel type of low toxicity solvents and can substitute solvent such as toluene, xylene, ethyl acetate, butyl acetate, acetone or butanone in paints and adhesive industry, and is environmentally friendly green chemical products.
2, it is excellent methylating agent, carbonylation agent, hydroxymethylation agent and methoxylation agent and is a kind of chemical raw material of wide application.
3, it can be used as ideal substitute of highly toxic product such as phosgene, dimethyl sulfate, and methyl chloroformate.
4, it can be used for synthesizing polycarbonate, diphenyl carbonate, and isocyanate and so on.
5, in the field of medicine, it can be for the synthesis of anti-infective drugs, anti-inflammatory medicines, vitamin-class medicines and central nervous system drugs.
6, in the field of pesticides, it can be mainly used for the production of methyl isocyanate, thereby producing some carbamate drugs, pesticides (anisole).
7, it can be used as gasoline additives and lithium battery electrolyte and so on.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| [Chemical Properties]
It is colorless liquid with a pungent odor. It is insoluble in water and soluble in alcohol, ether and other organic solvents.
| [Uses]
Dimethyl carbonate product can be used as traditional substitute of toxic materials phosgene, dimethyl sulfate and methyl chloride and so on. It can be used for synthesis of polycarbonate, diphenyl carbonate, isocyanate and allyl diglycol carbonate ester; it can also used for the synthesis of various kinds of carbamate pesticides such as carbaryl and so on; it can also be used as intermediate of organic synthesis such as anisole, dimethoxybenzene, alkylated aryl amines, symmetrical diamine urea, methyl carbazate and so on; in the pharmaceutical industry, it can be used for making amino oxazolidinone, ciprofloxacin, β-keto acid ester class pharmaceutical intermediates. In addition, it can be used as additives of gasoline, diesel fuel, the refrigerator oil and solvent.
| [Production method]
It can be produced through the reaction between methyl chloroformate ([79-22-1]) and methanol. The raw material, methyl chloroformate is produced from the reaction between methanol and phosgene. For the preparation, it is also plausible to have this phosgenation product been without isolation and add excess methanol for reflux reaction to synthesize dimethyl carbonate. The above reaction is called conventional phosgene method. 2 Transesterification methods: this is based on the transesterification between ethylene carbonate or propylene carbonate and methanol which can also produce dimethyl carbonate. This method has a high yield, small equipment corrosion and mild reaction conditions. However, the source of raw materials is limited by the development of petrochemical industry and the elements utilization rate is low. 3. Oxidative carbonylation method: this is based on the reaction of methanol, carbon monoxide and oxygen in the catalyst for direct synthesis of dimethyl carbonate. This method has a lot of advantages including easily available and cheap raw material, low toxicity and simple process. Therefore, it is the most promising approach. According to the technology conditions, it can be divided into liquid phase method and gas phase method.
Gas phase can be further divided into one-step and two-step method, wherein the liquid phase of methanol oxidation-carbonylation method and the gas-phase oxidative carbonylation step method has been industrialized while the one-step way of gas-phase oxidative-carbonylation of methanol is still in development. 4. The synthesis reaction between methanol and CO2. This process route is still in development. 5. Synthesis method via reaction between methanol and urea. This process route is still in development.
| [Category]
Flammable liquid
| [Toxicity grading]
Low toxicity
| [Acute toxicity]
Oral-rat LD50: 13000 mg/kg; Oral-Mouse LD50: 6000 mg/kg.
| [Flammability and hazard characteristics]
It is flammable in case of fire, high temperature and oxidant with burning causing irritated fume.
| [Storage characteristics]
Treasury: ventilation, low-temperature and dry; store it separately from oxidants.
| [Extinguishing agent]
Dry powder dry sand, carbon dioxide, foam, 1211 fire extinguishing agent. |
|
|