| Identification | More | [Name]
Nitrofurantoin | [CAS]
67-20-9 | [Synonyms]
1-(((5-nitro-2-furanyl)methylene)amino)-2,4-imidazolidinedione
1-(5-NITRO-2-FURFURYLIDENEAMINO)HYDANTOIN
1-[(5-nitrofurfurylidene)amino]hydantoin
FURADOXYL
LABOTEST-BB LT00134625
N-(5-NITRO-2-FURFURYLIDENE)-1-AMINOHYDANTOIN
NITROFURANTOIN
NITROFURANTOINE
1-(((5-nitro-2-furanyl)methylene)amino)-4-imidazolidinedione
1-((5-nitrofurfurylidene)amino)-hydantoi
1-([(E)-(5-Nitro-2-furyl)methylidene]amino)-2,4-imidazolidinedione
1-(5-Nitro-2-furfurylidenamino)hydantoin
1-[[(5-nitro-2-furanyl)methylene]amino]-4-imidazolidinedione
2,4-Imidazolidinedione, 1-[[(5-nitro-2-furanyl)methylene]amino]-
5-Nitrofurantoin
5-Nitrofurantoindorn
Benkfuran
berkfuran
Berkfurin
Chemiofuran | [EINECS(EC#)]
200-646-5 | [Molecular Formula]
C8H6N4O5 | [MDL Number]
MFCD00003224 | [Molecular Weight]
238.16 | [MOL File]
67-20-9.mol |
| Chemical Properties | Back Directory | [Appearance]
lemon yellow crystalline powder | [Melting point ]
268°C | [Boiling point ]
380.75°C (rough estimate) | [density ]
1.5824 (rough estimate) | [refractive index ]
1.6700 (estimate) | [storage temp. ]
0-6°C | [solubility ]
DMF: soluble50mg/mL | [form ]
crystalline
| [pka]
7.2(at 25℃) | [color ]
yellow
| [Stability:]
Stability Stable, but light-sensitive. Combustible. Incompatible with strong oxidizing agents, strong alkalies, strong acids. Decomposes upon contact with most metals other than stainless steel and aluminium. | [Water Solubility ]
<0.01 g/100 mL at 19 ºC | [Sensitive ]
Light Sensitive & Hygroscopic | [λmax]
358nm(MeOH)(lit.) | [Merck ]
13,6632 | [BRN ]
893207 | [BCS Class]
2 | [InChI]
1S/C8H6N4O5/c13-6-4-11(8(14)10-6)9-3-5-1-2-7(17-5)12(15)16/h1-3H,4H2,(H,10,13,14)/b9-3+ | [InChIKey]
NXFQHRVNIOXGAQ-YCRREMRBSA-N | [SMILES]
[O-][N+](=O)c1ccc(\C=N\N2CC(=O)NC2=O)o1 | [CAS DataBase Reference]
67-20-9(CAS DataBase Reference) | [IARC]
3 (Vol. 50) 1990 | [NIST Chemistry Reference]
Hydantoin, n-(5-nitro-2-furfurylidene)-1-amino-(67-20-9) | [EPA Substance Registry System]
67-20-9(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
S22:Do not breathe dust .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
2811 | [WGK Germany ]
3
| [RTECS ]
MU2800000
| [TSCA ]
Yes | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29349990 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral
Resp. Sens. 1
Skin Sens. 1 | [Safety Profile]
Poison by ingestion and
intraperitoneal routes. Human systemic
effects: peripheral motor nerve recording changes, ataxia, changes in urine
composition, and hemolysis with or without
anemia. Human reproductive effects by
ingestion: spermatogenesis. An experimental
teratogen. Other experimental reproductive
effects. Questionable carcinogen with
experimental neoplastigenic data. Human
mutation data reported. When heated to
decomposition it emits toxic fumes of NOx. | [Hazardous Substances Data]
67-20-9(Hazardous Substances Data) | [Toxicity]
LD50 oral in rat: 604mg/kg |
| Hazard Information | Back Directory | [General Description]
Odorless lemon yellow crystals or fine yellow powder. Bitter taste. | [Reactivity Profile]
NITROFURANTOIN(67-20-9) is sensitive to light. This chemical is incompatible with alkalis. NITROFURANTOIN(67-20-9) is also incompatible with strong oxidizers and strong acids. NITROFURANTOIN(67-20-9) decomposes on contact with metals other than stainless steel and aluminum. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Questionable carcinogen. | [Fire Hazard]
Flash point data for this chemical are not available; however, NITROFURANTOIN is probably combustible. | [Chemical Properties]
lemon yellow crystalline powder | [Originator]
Furadantin,Norwich Eaton ,US,1953 | [Uses]
A nitrofuran antibiotic with low resistance potential that is rapidly metabolized by mammals. Active against both Gram-positive and Gram-negative bacteria. Nitrofurantoin is also a prooxidant that is
cytotoxic due to the generation of intracellular H2O2. Antibacterial. | [Uses]
counterirritant | [Definition]
ChEBI: An imidazolidine-2,4-dione that is hydantoin substituted at position 1 by a [(5-nitro-2-furyl)methylene]amino group. An antibiotic that damages bacterial DNA. | [Indications]
Like nitrofurazone, nitrofurantoin is an effective drug that acts on a number of Gram�positive and Gram-negative microorganisms (staphylococci, streptococci, dysentery bacil�lus, colon bacillus, paratyphoid bacillus, and others). It is primarily used for treating infec�tious diseases of the urinary tract (pyelitis, pyelonephritis, cystitis, urethritis). Synonyms of
this drug are furadonin, ituran, phenurin, urolong, cistofuran, nitrofurin, and many others. | [Manufacturing Process]
To a solution of 18.9 grams (0.166 mol) n-heptaldehyde in 25 ml of isopropanol is added, with stirring, a solution of 19.1 grams (0.166 mol) of 1aminohydantoin in 110 ml water acidified with concentrated HCl. The heavy white precipitate formed is filtered and washed, until acid free, with small amounts of water and ether. The yield of N-(n-heptylidene)-1-aminohydantoin is 14 grams of MP 150°C (with decomposition). This may be recrystallized from dimethylformamide.
A mixture of 2.5 grams (0.016 mol) of 5-nitro-2-furaldoxime, 3.9 grams (0.018 mol) of N-(n-heptylidene)-1-aminohydantoin and 5 cc of sulfuric acid (density 1.84) is placed in a 250 cc beaker. It is heated with stirring at steam bath temperature for about 1.5 hours. Upon cooling, a solid precipitates which is collected by filtration, washed with water, isopropanol and ether in turn and dried at 110°C for 4 hours. There is obtained N-(5-nitro-2-furfurylidene)-1aminohydantoin in 96 to 98% yield, according to US Patent 2,927,110.
| [Brand name]
Ivadantin (Procter & Gamble);Furan;Nitrofan. | [Therapeutic Function]
Antibacterial (urinary) | [Antimicrobial activity]
It is active against almost all the common urinary
pathogens, except Proteus mirabilis. It is bactericidal.
It antagonizes the activity of nalidixic acid and other quinolones
in vitro, but this combination is unlikely to be used
clinically. | [Acquired resistance]
Surprisingly for an agent that has been used for so long, resistance
remains uncommon. R-factor-mediated resistance has
been reported, but this appears to be very unusual. The mechanism
of resistance seems to be a decreased nitroreductase
activity in the target organism.
There is cross-resistance within the nitrofuran group, but
none with antibiotics of other chemical classes. | [Pharmaceutical Applications]
A synthetic compound available only for oral administration.
There are three formulations, differing in their crystalline
nature: microcrystalline, macrocrystalline, and a delayedrelease
preparation containing a combination of the two. The
macrocrystalline form is said to be less liable to give rise to
the most common adverse event, nausea. However, pharmacokinetic
and clinical trial evidence for this assertion is not
very strong.
It is slightly soluble in water (c. 200 mg/L) but more so
in dilute alkali. Solubility in ethanol is modest (500 mg/L),
but the compound dissolves very well in dimethylformamide
(80 g/L). If packaged in light-resistant containers and kept at
room temperature, it is stable for more than 5 years. The yellow
solution should be kept in the dark. | [Biochem/physiol Actions]
Nitrofurantoin is an antibactericidal compound that has been historically prepared by the reaction of 1-aminohydantoin sulfate and 5-nitro-2-furaldehyde diacetate. It shows activity against many Gram-positive and Gram-negative bacteria. Nitrofurantoin is effective against enterococci, staphylococci, streptococci, corneybacteria, many strains of Escherichia coli. Most strains of Proteus spp. and Pseudomonas aeurginosa are more resistant to this compound. Nitrofurantoin is activated by bacterial flavoproteins (nitrofuran reductase) to actively reduce reactive intermediates that modulate and damage ribosomal proteins or other macromolecules, such as DNA. This inhibits DNA, RNA, protein, and cell wall synthesis which causes cell death. Nitrofurantoin has a low resistance potential that is rapidly metabolized by mammals and is active against both Gram-positive and Gram-negative bacteria. It is also a pro-oxidant that is cytotoxic due to the generation of intracellular H 2O2. It is an inhibitor of glutathione reductase. Nitrofurantoin produces hepatotoxicity caused by the redox cycling of the nitro group and its radical anion which leads to oxidative stress. | [Pharmacokinetics]
Oral absorption:>95%
Cmax 100 mg oral: <2 mg/L after 1–4 h
Plasma half-life:0.5–1 h
Volume of distribution: 0.6 L/kg
Plasma protein binding: 60–70%
Absorption
It is absorbed mainly from the proximal small intestine and
the plasma peak concentration may not be achieved for as
long as 4 h. The recommendation to take the drug with food
may be motivated by reducing the incidence of nausea rather
than increasing bioavailability.
Bioavailability varies widely between different brands and
this may not be apparent from results of standard in-vitro
pharmaceutical tests. Therefore, different brands should not
be substituted unless therapeutic equivalence has been formally
established.
Distribution
Serum levels are low, owing to extensive metabolism and the
short plasma half-life. Tissue concentrations are too low for
adequate treatment of systemic infection, including pyelonephritis.
Negligible concentrations are found in breast milk
and only a small amount crosses the placenta.
Metabolism and excretion
About 20% of the dose is excreted in microbiologically
active form in the urine, sufficient to give inhibitory concentrations
against urinary pathogens for up to 6 h. With
reduced renal function (creatinine clearance <60 mL/min),
urinary excretion falls, and virtually ceases when creatinine
clearance is below 20 mL/min. This gives rise to the risk of
accumulation in the blood and inadequate urine levels. With
this proviso, it can be given to elderly patients. Infants over
the age of 3 months may also be treated, but in the absence
of a suitable suspension, and at the recommended dosage, a
6-month baby would need to be given one-tenth of a standard
50 mg tablet. | [Clinical Use]
Acute dysuria and frequency
Bacteriuria in pregnancy
Prophylaxis of recurrent cystitis (reduced dosage) | [Synthesis]
Nitrofurantoin, 1-(5-nitrofurfurylidenamino)hydantoin (33.3.5), is syn�thesized from hydrazinoacetic acid (33.3.2), which is synthesized by reacting chloroacetic
acid with hydrazine. Reacting hydrazinoacetic acid with potassium cyanate gives the semi�carbazidoacetic acid (33.3.3), which upon heating cyclizes into 1-aminoidantoin (33.3.4).
Reacting this with diacetylacetal of 5-nitrofurfurol gives the desired nitrofurantoin.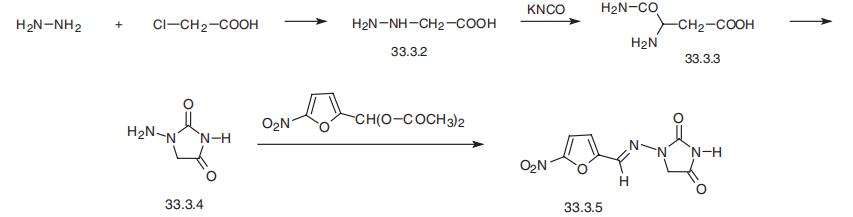
| [Veterinary Drugs and Treatments]
Considered a urinary tract antiseptic, nitrofurantoin is used primarily
in small animals, but also occasionally in horses in the treatment
of lower urinary tract infections caused by susceptible bacteria. It
is not effective in treating renal cortical or perinephric abscesses or
other systemic infections. | [Drug interactions]
Potentially hazardous interactions with other drugs
None known | [Metabolism]
Nitrofurantoin is metabolised in the liver and most body
tissues, while about 30-40% of a dose is excreted rapidly
in the urine as unchanged nitrofurantoin. Some tubular
reabsorption may occur in acid urine. |
| Questions And Answer | Back Directory | [Introduction]
Nitrofurantoin, (Furadantin, or N-(5-nitro-2-furfurylidene)-l-amino-hydantoin), is an antibiotic agent that fights bacteria in the body. Nitrofurantoin is a yellow, crystal-line compound of bitter taste, which darkens on exposure to light or alkali1. This antibiotic is commonly used to treat urinary tract infections. It is also extensively used in prophylaxis of uncomplicated lower urinary-tract infections2. Nitrofurantoin has been used since 1950s and it is a well-known and studied drug with limited antibiotic resistance3.
| [Medical use]
The oral dose of nitrofurantoin for adults is usually 50-100 mg, four times daily, with meals and at bedtime. Treatment of this medicine is usually continued for 14 days. The daily dose for children is 5-7 mg/kg, given in four divided oral doses. The dosage should be reduced if continued beyond 14 days or if used for prophylaxis. For long-term treatments, an oral dose of 1 mg/kg is recommended4.
| [Pharmacology and microbiology]
Nitrofurantoin has a wide range of antimicrobial activity against both Gram negative and positive organisms. Its bacteriostatic effect can be achieved at a concentration of 32 pg/ml especially in an acidic urine5. The mechanism of the antimicrobial action of nitrofurantoin is unusual among antibacterials. The nitro group coupled in the heterocyclic furan ring represents the specific active site of the drug and once activated by microbial nitro-reductases is able to interfere with protein and DNA synthesis, thus impairing energy metabolism, cell wall and carbohydrate synthesis. The broad-based nature of this action may explain the lack of acquired bacterial resistance to nitrofurantoin, as the necessary multiple and simultaneous mutations of the target macromolecules would likely be lethal to the bacteria 6. Nitrofurantoin is highly effective against E. coli, Enterococci, Aerobacter, and Staphylococcus saprophyticus infections, but less effective against Streptococcus, Proteus and Pseudornonas Serratia species7, 8.
From a pharmacokinetic point of view, the bioavailability of nitrofurantoin is about 90%. However, the plasma concentrations are very low, which is lower than 1 mg/L after an oral dose of 100 mg. The elimination half-life is short, which is around 1 h, and 27–50% of the nitrofurantoin drug is excreted unchanged in the urine. Even though peak urinary levels might be higher than 100 mg/L (range 50–200 mg/L), these are maintained for a relatively short time. Thus, nitrofurantoin should be avoided in patients with moderate to severe renal failure (creatinine clearance <50 mL/min)9.
| [Production]
Nitrofurantoin is not known to occur naturally. It can be prepared from 1-aminohydantoin sulfate or hydrochloride and 5-nitro-2-furaldehyde diacetate in isopropyl alcohol media10.
| [Side effects]
The common side effects of nitrofurantoin happen in more than 1 in 100 people. Nitrofurantoin may cause the patients’ urine turn into dark yellow or a brownish color. This symptom is normal and will disappear after stopping taking nitrofurantoin. Mild side effects of nitrofurantoin include feeling sick and vomiting, diarrhea, loss of appetite, headaches, dizziness or feeling sleepy. Serious side effects are rare and happen in less than 1 in 1,000 people. Those symptoms include but not limited to chest pains, difficulty in breathing, coughing, chills, or a high temperature, yellowing of the skin or eyes, tingling sensations, bleeding and bad headache. In emergency cases, it is also possible to have a serious allergic reaction to nitrofurantoin11.
Nitrofurantoin is classified in FDA pregnancy category B. It is not expected to be harmful to an unborn baby during early pregnancy 5, but it is not recommended for the last 2-4 weeks of pregnancy. Nitrofurantoin can pass into breast milk and may harm a nursing baby12. Nitrofurantoin should not be given to a child younger than 1 month old and patients having severe kidney diseases, urination problems or a history of jaundice or liver problems caused by nitrofurantoin13.
Hepatic injury after both acute and chronic exposure to nitrofurantoin has been reported. Long-term exposure to nitrofurantoin may cause chronic active hepatitis14.
Adverse reactions related to using nitrofurantoin in children reported in the literature are gastrointestinal disturbance, cutaneous reactions, pulmonary toxicity, hepatoxicity, hematological toxicity, neurotoxicity and an increased rate of sister chromatid exchanges. However, the use of nitrofurantoin is safe in children for long-term prophylactic therapy. Adverse reactions exist but they are less common than seen in adults, presumably because of the lower dose used for therapy, and the lack of significant comorbidities and drug interactions in children15.
Other side effects of nitrofurantoin have been associated with neurotoxicity (including peripheral neuropathy, dizziness, vertigo, diplopia, and cerebellar dysfunction) and benign intracranial hypertension. Those mentioned side effects are most prevalent in women and elderly patients, and their pathogenesis is hypothesized to be due to axon loss16.
| [Reference]
- H.B. Hasen, T.D. Moore, Nitrofurantoin: a study in vitro and in vivo in one hundred cases of urinary infection, Journal of the American Medical Association, 155(1954) 1470-3.
- A. Huttner, E.M. Verhaegh, S. Harbarth, A.E. Muller, U. Theuretzbacher, J.W. Mouton, Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials, Journal of Antimicrobial Chemotherapy, 70(2015) 2456-64.
- J.R. Price, L.A. Guran, W.T. Gregory, M.S. McDonagh, Nitrofurantoin vs other prophylactic agents in reducing recurrent urinary tract infections in adult women: a systematic review and meta-analysis, American journal of obstetrics and gynecology, 215(2016) 548-60.
- https://monographs.iarc.fr/wp-content/uploads/2018/06/mono50-15.pdf
- S.B. David, T. Einarson, Y.B. David, I. Nulman, A. Pastuszak, G. Koren, The safety of nitrofurantoin during the first trimester of pregnancy: meta‐analysis, Fundamental & clinical pharmacology, 9(1995) 503-7.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
- J.A. McKinnnel, L.G. Miller, Nitrofurantoin: Preferred Empiric Therapy for Community-Acquired Lower Urinary Tract Infections: In Response, Mayo Clinic Proceedings, Elsevier2011, p. 1244.
- D. Kyabaggu, F. Ejobi, D. Olila, The sensitivities to first-line antibiotic therapy of the common urinary tract bacterial infections detected in urine samples at a hospital in metropolitan Kampala (Uganda), African health sciences, 7(2007).
- A. Novelli, E. Rosi, Pharmacological properties of oral antibiotics for the treatment of uncomplicated urinary tract infections, Journal of Chemotherapy, 29(2017) 10-8.
- https://monographs.iarc.fr/wp-content/uploads/2018/06/mono50-15.pdf
- https://beta.nhs.uk/medicines/nitrofurantoin/
- P.M. Gerk, R.J. Kuhn, N.S. Desai, P.J. McNamara, Active transport of nitrofurantoin into human milk, Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 21(2001) 669-75.
- https://www.sciencedaily.com/releases/2015/04/150427124627.htm
- G. Amit, P. Cohen, Z. Ackerman, Nitrofurantoin-induced chronic active hepatitis, IMAJ-RAMAT GAN-, 4(2002) 184-6.
- E. Karpman, E.A. Kurzrock, Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children, The Journal of urology, 172(2004) 448-53.
- A. Mattappalil, K.A. Mergenhagen, Neurotoxicity with antimicrobials in the elderly: a review, Clinical therapeutics, 36(2014) 1489-511. e4.
|
|
|