| Identification | Back Directory | [Name]
N-Acetylmuramyl-alanyl-isoglutaminyl-alanyl-sn-glycero-3-phosphoethano lamine | [CAS]
83461-56-7 | [Synonyms]
MTP-PE
CGP 19835
MLV 19835
MIFAMURTIDE
MTP-cephalin
L-MTP-PE. MTP-PE
CGP 19835A Lipid
Mifamurtide (CGP-19835)
muramyl tripeptide phosphatidylethanolamine
muramyl tripeptide phosphatidylethanolamine liposome
N-Acetylmuramyl-alanyl-isoglutaminyl-alanyl-sn-glycero-3-phosphoethano lamine
N-Acetylmuramyl-alanyl-isoglutaminyl-alanyl-sn-glycero-3-phosphoethano lamine USP/EP/BP
CGP 19835A LIPID;MURAMYL TRIPEPTIDE PHOSPHATIDYLETHANOLAMINE LIPOSOME;L-MTP-PE;CGP-19835;MTP-PE;MTP-CEPHALIN;CGP19835;L-MTP-PE;MLV19835
N-(N-Acetylmuramoyl)-L-alanyl-D-α-glutaminyl-N-[(7R)-4-hydroxy-4-oxido-10-oxo-7-[(1-oxohexadecyl)oxy]-3,5,9-trioxa-4-phosphapentacos-1-yl]-L-alaninamide
L-Alaninamide, N-(N-acetylmuramoyl)-L-alanyl-D-α-glutaminyl-N-[(7R)-4-hydroxy-4-oxido-10-oxo-7-[(1-oxohexadecyl)oxy]-3,5,9-trioxa-4-phosphapentacos-1-yl]-
N-(N-Acetylmuramoyl)-L-alanyl-D-alpha-glutaminyl-N-[(7R)-4-hydroxy-4-oxido-10-oxo-7-[(1-oxohexadecyl)oxy]-3,5,9-trioxa-4-phosphapentacos-1-yl]-L-alaninamide | [EINECS(EC#)]
253-368-1 | [Molecular Formula]
C59H109N6O19P | [MDL Number]
MFCD09954133 | [MOL File]
83461-56-7.mol | [Molecular Weight]
1237.52 |
| Chemical Properties | Back Directory | [density ]
1.152 | [storage temp. ]
?20°C | [solubility ]
water: soluble2mg/mL, clear (warmed) | [form ]
powder | [pka]
1.39±0.50(Predicted) | [color ]
white to beige | [Water Solubility ]
water: 2mg/mL, clear (warmed) | [InChIKey]
ZVLWUMPAHCEZAW-HYGHKABSNA-N | [CAS DataBase Reference]
83461-56-7 |
| Hazard Information | Back Directory | [Uses]
Osteosarcoma. | [Biochem/physiol Actions]
Mifamurtide is an immunomodulator and regulates the activation of monocytes and macrophages. Mifamurtide upregulates the secretion of pro-inflammatory cytokines such as TNF-α, IL-1, IL-8, nitric oxide and prostaglandins E2 and D2. It has anti-tumor effects in children and young adults with high-grade osteosarcoma. | [Mechanism of action]
Being a
phospholipid, mifamurtide accumulates in the lipid bilayer of the liposomes upon infusion. After
application of the liposomal infusion, the drug is cleared from the plasma within minutes. However, it is
concentrated in lung, liver, spleen, nasopharynx and thyroid, and the terminal half-life is 18 hours,
which is longer than the natural substance. | [Clinical Use]
Mifamurtide is an anticancer agent for the treatment of osteosarcoma, the most common primary
malignancy of bone tissue mainly affecting children and adolescents. The drug was invented by
Ciba-Geigy (now Novartis) in the early 1980s and the agent was subsequently licensed to Jenner
Biotherapies in the 1990s. IDM Pharma bought the rights to the drug from Jenner in April 2003.In March 2009, mifamurtide was approved in the 27 European Union member states plus Iceland,
Liechtenstein and Norway via a centralized marketing authorization. After the approval, IDM Pharma
was acquired by Takeda, which began launching mifamurtide, as Mepact ®, in February 2010.
Mifamurtide, a fully synthetic lipophilic derivative of muramyl dipeptide (MDP), is muramyl tripeptide
phosphatidylethanolamine (MTP-PE), which is formulated as a liposomal infusion. | [Side effects]
Mifamurtide is generally well tolerated; adverse events attributed to its administration include chills, fever, headache, nausea, and myalgias[1].
| [Synthesis]
Two synthetic routes have been reported, and
the scheme describes the more process-amenable route. Commercially available 1,2-dipalmitoyl-snglycero-
3-phosphoethanolamine (110) was coupled with N-Boc-L-alanine (111) by means of Nhydroxysuccinimide
(112), DCC in DMF to give amide 113, which was followed by hydrogenolysis of
the CBZ group to give the corresponding L-alanyl-phosphoric acid 114. Next, commercially available
N-acetylmuramoyl-L-alanyl-D-isoglutamine (115) was subjected to hydroxybenzotriazole (HOBT) and
DIC in DMF to provide the corresponding succinimide ester 116 which was condensed with compound
114 to provide mifamurtide (IX). No yields were provided for these transformations.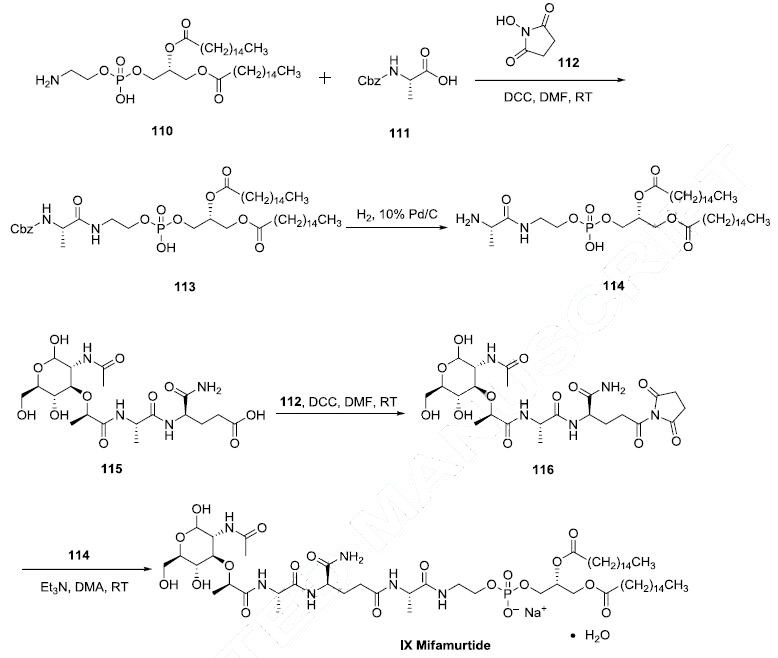
| [Drug interactions]
Potentially hazardous interactions with other drugs
Analgesics: avoid with high dose NSAIDs.
Ciclosporin: avoid concomitant use.
Corticosteroids: avoid concomitant use.
Tacrolimus: avoid concomitant use. | [Metabolism]
The cells of the reticuloendothelial system clear
mifamurtide liposomes by phagocytosis. | [storage]
Store at -20°C | [References]
[1] Frampton, James E. “Mifamurtide: a review of its use in the treatment of osteosarcoma.” Paediatric drugs vol. 12,3 (2010): 141-53. doi:10.2165/11204910-000000000-00000
|
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
| Company Name: |
NCE Biomedical Co.,Ltd.
|
| Tel: |
4000-027-021 |24 +86-13986109188 | +86-15623472865 | +81-08033611988 |
| Website: |
www.chemicalbook.com/ShowSupplierProductsList15748/0_EN.htm |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|