| Identification | More | [Name]
Lamotrigine | [CAS]
84057-84-1 | [Synonyms]
3,5-DIAMINO-6-(2,3-DICHLOROPHENYL)-1,2,4-TRIAZINE
6-(2,3-DICHLOROPHENYL)-1,2,4-TRIAZINE-3,5-DIAMINE
BW-430C
GI 267119X
LAMICTAL
LAMOTRIGIN
LAMOTRIGINE
LAMOTRIGINE-13C1
LTG
4-triazine-3,5-diamine,6-(2,3-dichlorophenyl)-2
Lemotrigine
(2,3-Dichloro Phenyl)-1,2,4-triazine-3,5-diamine
6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine, LTG, BW-430C, Lamictal
1,2,4-Triazine-3,5-diamine, 6-(2,3-dichlorophenyl)-
LAMOTRINGINE
6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine, GI 267119X
BW-430
Lamotrigine 6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine | [EINECS(EC#)]
281-901-8 | [Molecular Formula]
C9H7Cl2N5 | [MDL Number]
MFCD00865333 | [Molecular Weight]
256.09 | [MOL File]
84057-84-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White to Cream Coloured Powder | [Melting point ]
177-181°C | [Boiling point ]
503.1±60.0 °C(Predicted) | [density ]
1.572±0.06 g/cm3(Predicted) | [Fp ]
9℃ | [storage temp. ]
2-8°C
| [solubility ]
DMSO: 20 mg/mL at 60 °C, soluble
| [form ]
powder
| [pka]
5.7(at 25℃) | [color ]
white
| [Usage]
An anticonvulsant. Inhibits glutamate release, possible through inhibition of Sodium, Potassium and Calcium currents | [Merck ]
14,5353 | [BCS Class]
2 | [InChI]
InChI=1S/C9H7Cl2N5/c10-5-3-1-2-4(6(5)11)7-8(12)14-9(13)16-15-7/h1-3H,(H4,12,13,14,16) | [InChIKey]
PYZRQGJRPPTADH-UHFFFAOYSA-N | [SMILES]
N1C(C2=CC=CC(Cl)=C2Cl)=C(N)N=C(N)N=1 | [CAS DataBase Reference]
84057-84-1(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi | [Risk Statements ]
R25:Toxic if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
XY5850700
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29336990 | [Hazardous Substances Data]
84057-84-1(Hazardous Substances Data) | [Toxicity]
LD50 in mice, rats (mg/kg): 250, >640 orally (Sawyer) |
| Hazard Information | Back Directory | [Description]
Lamotrigine, also known by the brand name Lamictal®, is a second-generation antiepileptic drug (AED) manufactured by GlaxoSmithKline in the UK and USA. Lamotrigine is a new mazine, glutamate inhibitor anticonvulsant that significantly reduces the incidence of refractory partial seizures. The drug is reported to produce fewer CNS side effects than diazepam or sodium phenytoin. It is also indicated as add-on therapy for the treatment of generalized seizures not satisfactorily controlled by other anti-epileptics. | [Chemical Properties]
White to Cream Coloured Powder | [Originator]
Burroughs Wellcome (United Kingdom) | [History]
The development of lamotrigine began in the early 1980s, accidentally discovered by a group of chemists at the Wellcome Research Laboratory while searching for novel antifolate compounds. Initially synthesized as an antifolate analog, subsequent animal model screening revealed its significant anticonvulsant activity, demonstrating its potential as an antiepileptic drug. In 1980, the Wellcome Foundation filed the first synthetic method and related patent applications for lamotrigine (e.g., European Patent EP 0021121), mentioning researchers such as M. G. Baxter. During clinical development, the efficacy of lamotrigine (trade name: lamotrigine tablets) was confirmed, and it was first launched in Ireland in 1991. Subsequently, it was approved by the U.S. Food and Drug Administration (FDA) for the treatment of epilepsy in 1994. In subsequent studies, the clinical applications of lamotrigine have continued to expand, particularly its efficacy as a mood stabilizer. Researchers such as Richard H. Weissler have advanced the application of lamotrigine in the treatment of bipolar disorder, especially in the maintenance treatment of bipolar depression, through clinical observation and research. | [Uses]
Lamotrigine is an anticonvulsant that works by Inhibits glutamate release, possibly through inhibition of Sodium, Potassium, and Calcium currents. Used in the treatment of bipolar depression, partial seizures in epilepsy, and generalized seizures of Lennox-Gastaut syndrome. Additionally, it is used for the maintenance treatment of bipolar I disorder and depression. | [Definition]
ChEBI: Lamotrigine is a member of the class of 1,2,4-triazines in which the triazene skeleton is substituted by amino groups at positions 3 and 5, and by a 2,3-dichlorophenyl group at position 6. It has a role as an anticonvulsant, an antimanic drug, an antidepressant, a non-narcotic analgesic, a calcium channel blocker, an excitatory amino acid antagonist, an EC 3.4.21.26 (prolyl oligopeptidase) inhibitor, an environmental contaminant, a xenobiotic and a geroprotector. It is a member of 1,2,4-triazines, a primary arylamine and a dichlorobenzene. | [Preparation]
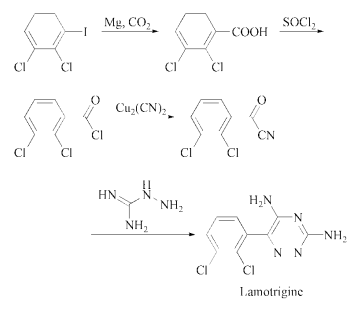
The preparation method of Lamotrigine involves several steps. 2,3-dichlorobenzoic acid is chlorinated to 2,3-dichlorobenzoyl chloride, then reacted with cuprous cyanide, condensed with aminoguanidine, and finally cyclized under the action of potassium hydroxide Lamotrigine.
Two key methods for the synthesis of lamotrigine have been reported.
https://www.sciencedirect.com/topics/chemistry/lamotrigine
A novel process for the synthesis of lamotrigine and its intermediate
https://patents.google.com/patent/WO2007069265A1/en | [Brand name]
Lamictal (Glax oSmithKline). | [Therapeutic Function]
Anticonvulsant | [World Health Organization (WHO)]
Lamotrigine is a relatively new antiepilepsy agent acting through
stabilization of neuronal membranes and preventing liberation of neurotransmitters. | [Biological Functions]
Lamotrigine has a broad spectrum of action and is effective in generalized and partial epilepsies. Its primary mechanism of action appears to be blockage of voltagedependent sodium channels, although its effectiveness against absence seizures indicates that additional mechanisms may be active. Lamotrigine is almost completely absorbed from the gastrointestinal tract, and peak plasma levels are achieved in about 2 to 5 hours. The plasma half-life after a single dose is about 24 hours. Unlike most drugs, lamotrigine is metabolized primarily by glucuronidation. Therefore, it appears likely that lamotrigine will not induce or inhibit cytochrome P450 isozymes, in contrast to most AEDs. | [General Description]
Lamotrigine(84057-84-1) is an antiepileptic drug belonging in the phenyltriazine class. It is used in the treatment of both epilepsy and as a mood stabilizer in bipolar disorder. Lamotrigine is the first medication since lithium granted Food and Drug Administration (FDA) approval for the maintenance treatment of bipolar type I. It is approved for use in more than 30 countries.
| [Biological Activity]
Anticonvulsant. Inhibits glutamate release, possibly through inhibition of Na + , K + and Ca 2+ currents. | [Mechanism of action]
Lamotrigine has been found effective against refractory partial seizures. Like phenytoin and CBZ, its main mechanism of action appears to be a blockade of sodium channels that is both voltage- and used-dependent. It also inhibits the high-threshold calcium channel, possibly through inhibition of presynaptic N-type calcium channels, and also blocks glutamate release. The most probable explanation for lamotrigine's efficacy is its ability to produce a blockade of sodium channel repetitive firing. In addition, lamotrigine appears to reduce glutaminergic excitatory transmission, although the mechanism for this action remains unclear. | [Pharmacokinetics]
Following oral administration, lamotrigine is absorbed rapidly and completely, exhibiting linear pharmacokinetics and modest
protein binding (55%). Lamotrigine is metabolized predominantly by N-glucuronidation and subsequent urinary elimination of its
major metabolite, the quaternary 2-N-glucuronide (80–90%), the minor 5-amino-N-glucuronide (8–10%), and unchanged drug
(8–10%). Lamotrigine's usual elimination half-life of 24–35 hours is reduced to 13–15 hours in patients taking enzyme�inducing AEDs. The presence of valproate increases the lamotrigine half-life substantially by inhibiting N-glucuronidation,
necessitating a reduction in dose to avoid toxicity. Hepatic disease patients may demonstrate a reduced capacity to for
lamotrigine glucuronidation, thus reducing its rate of clearance. | [Clinical Use]
Lamotrigine is a 5-phenyl-1,2,4-triazine derivative indicated as monotherapy or as an adjunct for partial seizures in adults, as
adjunct in patients with Lennox-Gastaut syndrome, and as adjunct for partial seizures in children 2 years of age and older.
Lamotrigine may have additional benefit in combating myoclonic and typical absence seizures. It is approved for use in the
maintenance treatment of bipolar disorder. | [Side effects]
The usefulness of lamotrigine is limited by the increased incidence of serious rashes, particularly in children or patients taking
valproate. This increase, however, may be attenuated by very slow dose escalation, because most rashes appear within
the first 8 weeks of treatment. The drug should be discontinued if a rash appears at any time. Additionally, lamotrigine may be
associated with development of myoclonus after 2 to 3 years of drug treatment. Additional common side effects associated
with lamotrigine therapy include dizziness, diplopia, headache, ataxia, blurred vision, somnolence, and nausea. | [Synthesis]
The reaction of the Grignard compound
of 2,3-dichloroiodobenzene with CO2
in diethyl ether gives 2,3-dichlorobenzoic acid,
which is converted to the corresponding acyl
chloride by refluxing with SOCl2. The reaction
of 2,3-dichlorobenzoyl chloride with
cuprous cyanide and KI in refluxing xylene
yields 2,3-dichlorobenzoyl cyanide. Finally, this
compound is cyclized with aminoguanidine in
DMSO to yield lamotrigine .
 | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin.
Antidepressants: antagonism of anticonvulsant
effect; avoid with St John’s wort.
Antiepileptics: concentration reduced by
carbamazepine, phenobarbital and phenytoin, also
possibility of increased concentration of active
carbamazepine metabolite; concentration increased
by valproate - reduce lamotrigine dose.
Antimalarials: mefloquine antagonises
anticonvulsant effect
Antipsychotics: anticonvulsant effect antagonised.
Oestrogens and progestogens: concentration of
lamotrigine reduced and the dose may need to
be increased by as much as 2-fold; may affect
contraceptive effect.
Orlistat: possibly increased risk of convulsions | [Metabolism]
Lamotrigine is extensively metabolised in the liver by
UDP-glucuronyl transferases and excreted almost entirely
in urine, principally as an inactive glucuronide conjugate.
It slightly induces its own metabolism. Only about 2% of
lamotrigine-related material is excreted in faeces. | [storage]
Room temperature | [References]
https://my.clevelandclinic.org/health/drugs/20217-lamotrigine-tablets
https://pubchem.ncbi.nlm.nih.gov/compound/Lamotrigine
https://go.drugbank.com/drugs/DB00555
The_Renal_Drug_Handbook_The_Ultimate |
|
|