| Identification | More | [Name]
N-Boc-2-piperidone | [CAS]
85908-96-9 | [Synonyms]
1-BOC-1-AZACYCLOHEXAN-2-ONE
1-BOC-2-PIPERIDONE
1-N-BOC-2-PIPERIDONE
N-BOC-2-PIPERIDONE
N-BOC-DELTA-VALEROLACTAM
N-(TERT-BUTOXYCARBONYL)-2-PIPERIDONE
N-(TERT-BUTYLOXYCARBONYL)-2-PIPERIDONE
1-(tert-Butoxycarbonyl)-2-piperidone~tert-Butyl 2-oxo-1-piperidinecarboxylate
1-Boc-2-pieridone
1-(Tert-butoxycarbonyl)-2-piperidone
Tert-butyl-2-oxo-1-piperidinecarboxylate
1-Piperidinecarboxylic acid, 2-oxo-, 1,1-dimethylethyl ester
1-BOC-2-OXOPIPERIDINE
tert-butyl 2-oxopiperidine-1-carboxylate | [Molecular Formula]
C10H17NO3 | [MDL Number]
MFCD02179046 | [Molecular Weight]
199.25 | [MOL File]
85908-96-9.mol |
| Chemical Properties | Back Directory | [Melting point ]
29-36 °C | [Boiling point ]
110°C/0.1mmHg(lit.) | [density ]
1.099±0.06 g/cm3(Predicted) | [Fp ]
>110 °C | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
Chloroform, Methanol | [form ]
Solid | [pka]
-2.29±0.20(Predicted) | [color ]
Off-White | [BRN ]
4674955 | [InChI]
InChI=1S/C10H17NO3/c1-10(2,3)14-9(13)11-7-5-4-6-8(11)12/h4-7H2,1-3H3 | [InChIKey]
ULMHMJAEGZPQRY-UHFFFAOYSA-N | [SMILES]
N1(C(OC(C)(C)C)=O)CCCCC1=O | [CAS DataBase Reference]
85908-96-9(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
N | [Risk Statements ]
R50:Very Toxic to aquatic organisms. | [Safety Statements ]
S24/25:Avoid contact with skin and eyes .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 3082 | [WGK Germany ]
3 | [HazardClass ]
IRRITANT | [HS Code ]
29337900 |
| Hazard Information | Back Directory | [Chemical Properties]
Off-White Low Melting Solid | [Uses]
N-Boc-2-piperidone(85908-96-9) is used as a synthetic intermediate in the preparation of various biological inhibitors. | [Definition]
ChEBI: Piperidin-2-one N-protected by t-Boc. | [Preparation]
4-Benzensulfonylmethyl-4-hydroxy-piperidine TFA (B-1) Diisopropylethylamine (28.3 mL, 162.75 mmol) and di-t-butyl dicarbonate (28.4 g, 130.02 mmol) were added in sequence to a mixture of piperidone hydrate hydrochloride (12) (10.0 g, 65.1 mmol) in methanol (50 mL). The mixture was stirred at room temperature for 20 hours. After that, removed the solvent and the remaining was separated with ether and 1 M KHSO4 solution. The organic layer was washed with brine and saturated NaHCO3. Finally, N-Boc-2-piperidone was obtained after purification.
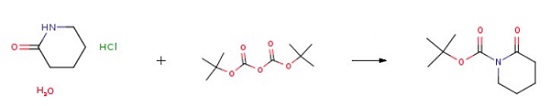 | [Synthesis]
To a solution of piperidin-2-one (0.97 g, 9.78 mmol) in dichloromethane (DCM, 20 mL) was sequentially added triethylamine (Et3N, 1.36 mL, 9.78 mmol), 4-dimethylaminopyridine (DMAP, 0.12 g, 0.978 mmol) and di-tert-butyl dicarbonate (Boc2O, 3.20 g, 14.7 mmol ). The reaction mixture was stirred at room temperature for 3 h. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was purified by silica gel column chromatography with the eluent ethyl acetate/petroleum ether (v/v = 1/7) to afford 1-Boc-2-piperidone as a light yellow oil (1.78 g, 91% yield). Mass spectrum (ESI, cation mode) m/z: 144.2 [(M-C4H8)+ H]+; 1H NMR (600 MHz, CDCl3): δ (ppm) 3.65 (t, J = 6.1 Hz, 2H), 2.50 (t, J = 9.6, 7.2 Hz, 2H), 1.82 (m, 4H), 1.52 (s, 9H). | [References]
[1] Journal of the American Chemical Society, 2008, vol. 130, # 23, p. 7449 - 7458
[2] Organic and Biomolecular Chemistry, 2016, vol. 14, # 31, p. 7585 - 7593
[3] Heterocycles, 2009, vol. 77, # 1, p. 417 - 432
[4] Patent: WO2015/94803, 2015, A1. Location in patent: Paragraph 395
[5] Patent: WO2016/190847, 2016, A1. Location in patent: Paragraph 0359 |
|
|