| Identification | More | [Name]
Bortezomib | [CAS]
179324-69-7 | [Synonyms]
[(1r)-3-methyl-1-[[(2s)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]-boronic acid
BORTEZOMIB
dpba
VELCADE
VELCADE(BORTEZOMIB)
Boronic acid, B-[(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(2-pyrazinylcarbonyl)amino]propyl]amino]butyl]-
Bortezomib for research | [EINECS(EC#)]
605-854-3 | [Molecular Formula]
C19H25BN4O4 | [MDL Number]
MFCD05260688 | [Molecular Weight]
384.24 | [MOL File]
179324-69-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Yellow Solid | [Melting point ]
122-124°C | [density ]
1.214 | [RTECS ]
ED7771666 | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [solubility ]
Soluble in chloroform, dimethyl sulfoxide, ethanol and methanol. | [form ]
solid | [pka]
9.66±0.43(Predicted) | [color ]
White | [Optical Rotation]
Consistent with structure | [Usage]
Bortezomib is the first proteasome inhibitor to be approved b the US FDA for multiple myeloma, a blood cancer. A reversible inhibitor of the 26S proteasome-a barrel-shaped multiprotein particle found in the nucleus and cytosol of all eukaryotic cel | [Stability:]
Hygroscopic and Moisture Sensitive | [InChIKey]
GXJABQQUPOEUTA-RDJZCZTQSA-N | [SMILES]
B([C@@H](NC(=O)[C@@H](NC(C1=NC=CN=C1)=O)CC1=CC=CC=C1)CC(C)C)(O)O | [CAS DataBase Reference]
179324-69-7(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [Description]
Bortezomib, a modified dipeptidyl boronic acid, is a therapeutic proteasome inhibitors used for the treatment of cancers. It is indicated for the treatment of relapsed multiple myeloma and mantle cell lymphoma. It is capable of inhibiting the mammalian 26S proteasome, which is important in regulating the intracellular concentration of specific proteins to maintain homeostasis within cells. The disruption of 26S proteasome function disrupts normal cellular homeostasis, leading to cytotoxic effect on various kinds of cancer cells.
| [Drug for Cancer treatment]
Bortezomib is a drug for treatment of hematopoietic malignancies with the appearance being white or white-like crystalline powder. It is easily soluble in dimethyl sulfoxide, ethanol, but insoluble in aqueous solution. This product is the reversible inhibitor of the mammal cell 26S proteasome chymotrypsin-like activity. 26S proteasome is a large protein complex which can degrade ubiquitin. Ubiquitin proteasome pathway plays an important role in regulation of the intracellular concentration of specific proteins in order to maintain the stability of the intracellular environment. Proteolytic affects intracellular multi-level signalling cascade. The disruption of the normal intracellular environment can lead to cell death while the inhibition of the 26S proteasome can prevent the hydrolysis of specific proteins. In vitro tests have proved bortezomib exhibits cytotoxicity to multiple types of cancer cells. The in vivo models of preclinical tumor have proved that bortezomib is capable of delaying the tumor growth of multiple myeloma which is suitable for the treatment of multiple myeloma.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
|
| Hazard Information | Back Directory | [Chemical Properties]
Yellow Solid | [Originator]
Millenium
(LeukoSite, Proscript) (US) | [Uses]
Bortezomib is the first proteasome inhibitor to be approved b the US FDA for multiple myeloma, a blood cancer. A reversible inhibitor of the 26S proteasome-a barrel-shaped multiprotein particle found in the nucleus and cytosol of all eukaryotic cells. T | [Definition]
ChEBI: L-Phenylalaninamide substituted at the amide nitrogen by a 1-(dihydroxyboranyl)-3-methylbutyl group and at Nalpha by a pyrazin-2-ylcarbonyl group. It is a dipeptidyl boronic acid tha
reversibly inhibits the 26S proteasome. | [Brand name]
Velcade
(Millennium). | [General Description]
Proteasomes normally function to degrade proteins that areno longer needed by the cell. Such proteins are normallymarked by the addition of ubiquitin, a 76 amino acid proteinthat is added to the -amino group of lysine residues onthe target proteins. The marked proteins are then hydrolyzedby the large barrel-shaped proteasomes to givepeptides of 7 to 8 residues that may be further hydrolyzedand reutilized by the cell. This process serves to regulateprotein levels within the cell, remove defective proteins,and becomes important in maintaining normal signal transduction.Inhibition of the proteasomes results in the buildup of ubiquitylated proteins, which disrupts cell-signalingprocesses and cell growth. The signaling bytranscription factor NF-B (nuclear factor B) appears tobe especially sensitive to bortezomib. NF-B is associatedwith the transcription of antiapoptotic and proliferativegenes but is under the control of IB (inhibitor of NF-B).IB can itself be phosphorylated by IKK (IB kinase),which marks IB for ubiquitylation and destruction allowingNF-B to mediate its antiapoptotic and proliferative effects effects.In the presence of the competitive inhibitor bortezomib(IC50=0.6 nM), the 26S proteasome is inhibitedand the ubiquitylated IkB is still capable of inhibiting NF-kB, preventing its effects. | [Pharmaceutical Applications]
Bortezomib belong to the class of drugs called proteasome inhibitors and is licensed in the United States and
the United Kingdom for the treatment of multiple myeloma. The drug has been licensed for patients in whom
the myeloma has progressed despite prior treatment or where a bone marrow transplant is not possible or was
not successful. It is marketed under the name Velcade? or Cytomib?. Velcade is administered via injection
and is sold as powder for reconstitution.
Bortezomib was the first drug approved in the new drug class of proteasome inhibitors and boron seems to
be its active element. For the mode of action, it is believed that the boron atom binds with high affinity and
specificity to the catalytic site of 26S proteasome and inhibits its action. Therapy with Bortezomib can lead
to a variety of adverse reactions, including peripheral neuropathy, myelosuppression, renal impairment and
gastrointestinal (GI) disturbances together with changes in taste. Nevertheless, the side effects are in most
cases less severe than with alternative treatment options such as bone marrow transplantation. | [Biochem/physiol Actions]
Cell permeable: yes | [Clinical Use]
Proteasome inhibitor:
Treatment of multiple myeloma for people who have
already tried at least 1 prior therapy and have disease
progression | [Synthesis]
Although the synthesis of dipeptidyl boronic acids
have appeared on several reports, the synthetic
details for bortezomib were not revealed. The synthetic route
for the preparation of bortezomib is depicted in the scheme.The pinanediol ester of leucine boronic acid (56)was
coupled with N-Boc phenylalanine (57) in the presence of
TBTU followed by deprotection of the Boc group to provide
58. N-Acylation of 58 then furnished the dipeptide boronate
ester 60. Deprotection of the boronic ester functionality was
achieved by bi-phase transfer esterification with isobutyl
boronic acid. Bortezomib (VI) was isolated by extractive
workup.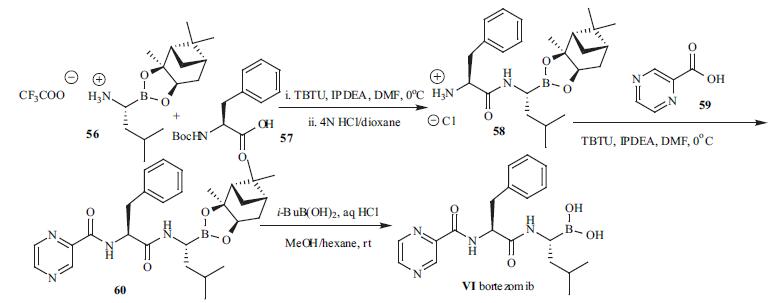
| [target]
20S proteasome | [Drug interactions]
In vitro studies with human liver microsomes and human
cDNA-expressed cytochrome P450 isozymes indicate
that bortezomib is primarily oxidatively metabolised via
cytochrome P450 enzymes, 3A4, 2C19, and 1A2. The major
metabolic pathway is deboronation to form two deboronated
metabolites that subsequently undergo hydroxylation to
several metabolites. Deboronated-bortezomib metabolites
are inactive as 26S proteasome inhibitors. | [Metabolism]
In vitro studies with human liver microsomes and human
cDNA-expressed cytochrome P450 isozymes indicate
that bortezomib is primarily oxidatively metabolised via
cytochrome P450 enzymes, 3A4, 2C19, and 1A2. The major
metabolic pathway is deboronation to form two deboronated
metabolites that subsequently undergo hydroxylation to
several metabolites. Deboronated-bortezomib metabolites
are inactive as 26S proteasome inhibitors. | [storage]
+4°C | [References]
1) Adams et al. (1999), Proteasome inhibitors: a novel class of potent and effective antitumor agents; Cancer Res., 59 2615
2) Williams et al. (2003), Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts; Mol. Cancer Ther., 2 835
3) Richardson et al. (2003), Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers; Cancer Control, 10 361
4) Herve and Ibrahim (2017), Proteasome inhibitors to alleviate aberrant IKBKAP mRNA splicing and low IKAP/hELP1 synthesis in familial dysautonomia; Neurobiol. Dis., 103 113 |
|
|