106092-09-5
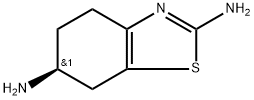 106092-09-5 结构式
106092-09-5 结构式
基本信息
(6S)-2,6-二氨基-4,5,6,7-四氢苯并噻唑
(6S)-4,5,6,7-TETRAHYDRO-1,3-BENZOTHIAZOLE-2,6-DIAMINE
S-(-)-2,6-Diamino-4,5,6,7-tetrahydrobenzothiazole
(s)-4,5,6,7-tetrahydro-2,6-benzothiazolediamine
(S)-4,5,6,7-TETRAHYDROBENZO[D]THIAZOLE-2,6-DIAMINE
(S)-4,5,6,7-TETRAHYDRO-BENZOTHIAZOLE-2,6-DIAMINE
(-)-2,6-diamino-4,5,6,7-tetrahydroben thiazole
(-)-(6S)-2,6-Diamino-4,5,6-tetrahydrobenzothiazole
S(-) 2-Amino-6-amino-4,5,6,7-tetrahydro benzothiazole
S-(-)-2,6-Diamino-4,5,6,7-tetrahydrobenzothiazole (6S)-2,6-Diamino-4,5,6,7-tetrahydrobenzothiazole
(6S)-2,6-Diamino-4,5,6,7-tetrahydrobenzothiazole
(-)-2,6-DIAMINO-4,5,6,7-TETRA-HYDROXYBENZOTHIAZOL
S-(-)-2,6-Diamino-4,5,6,7-tetrahydro-2,6-benzothiazole
(6S)-4,5,6,7-Tetrahydro-2,6-benzothiazolediamine
4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine
物理化学性质
制备方法
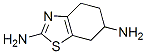
106006-83-1

106092-09-5
以2,6-二氨基-4,5,6,7-四氢苯并噻唑为原料合成(S)-2,6-二氨基-4,5,6,7-四氢苯并噻唑的一般步骤:首先,将33.8g 2,6-二氨基-4,5,6,7-四氢苯并噻唑与500mL水混合,随后加入30.0g L-(+)-酒石酸。将反应混合物加热至75°C并搅拌1小时。之后,冷却至室温并继续搅拌24小时。反应完成后,进行过滤,用水洗涤沉淀,并在55°C至65°C、-0.01MPa至-0.1MPa的真空条件下干燥8-12小时,得到35.1g (S)-2,6-二氨基-4,5,6,7-四氢苯并噻唑二L-(+)-酒石酸盐粗品,其对映体含量为2.53%(通过手性-HPLC测定)。将全部粗品重新溶解于500mL水中,加热至75°C保持1小时,然后冷却至室温并搅拌24小时。再次过滤,用水洗涤,并在相同真空条件下干燥8-12小时,得到26.9g (S)-2,6-二氨基-4,5,6,7-四氢苯并噻唑二L-(+)-酒石酸盐,其对映体含量降低至0.08%。最后,将全部产物溶解于200mL水和40mL盐酸的混合溶液中,搅拌至完全溶解。随后,滴加35滴氢氧化钾水溶液(质量百分比为氢氧化钾占溶液总质量的比例)进行中和。反应混合物冷却至室温后搅拌1小时,过滤,用水洗涤沉淀,并在55°C至65°C、-0.01MPa至-0.1MPa的真空条件下干燥8-12小时,最终得到9.43g (S)-2,6-二氨基-4,5,6,7-四氢苯并噻唑,收率为27.9%,对映体含量为0.08%。
参考文献:
[1] Journal of Medicinal Chemistry, 2015, vol. 58, # 14, p. 5501 - 5521
[2] Patent: CN108084115, 2018, A. Location in patent: Paragraph 0086; 0089
[3] Journal of Medicinal Chemistry, 1987, vol. 30, # 3, p. 494 - 498
[4] Patent: WO2011/21214, 2011, A2
[5] Investigational New Drugs, 2010, vol. 28, # 4, p. 454 - 465
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | D4337 | (S)-(-)-2,6-二氨基-4,5,6,7-四氢苯并噻唑 (S)-(-)-2,6-Diamino-4,5,6,7-tetrahydrobenzothiazole | 106092-09-5 | 1g | 95元 |
| 2025/12/22 | XW0210461749403 | 2,6-二氨基-4,5,6,7-四氢苯并噻唑(普拉克索中间体) 4,5,6,7-Tetrahydrobenzo[d]thiazole-2,6-diamine | 106092-09-5 | 25g | 171元 |
| 2025/12/22 | XW0210461749402 | 2,6-二氨基-4,5,6,7-四氢苯并噻唑(普拉克索中间体) 4,5,6,7-Tetrahydrobenzo[d]thiazole-2,6-diamine | 106092-09-5 | 10g | 88元 |
