109384-24-9
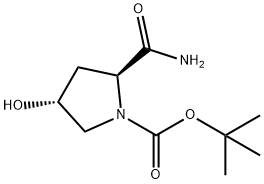 109384-24-9 结构式
109384-24-9 结构式
基本信息
(2S,4R)-1-BOC-2-氨基甲酰-4-羟基吡咯烷
(2S,4R)-1-BOC-2-氨基甲酰基-4-羟基吡咯烷
(2S,4R)-1-Boc-2-carbamoyl-4-hydroxypyrrolidine
-tert-Butyl 2-carbamoyl-4-hydroxypyrrolidine-1-carboxylate
tert-butyl (2S,4R)-2-carbamoyl-4-hydroxypyrrolidine-1-carboxylate
(2S,4R)-tert-butyl 2-carbamoyl-4-hydroxypyrrolidine-1-carboxylate
1-Pyrrolidinecarboxylic acid, 2-(aminocarbonyl)-4-hydroxy-, 1,1-dimethylethyl ester, (2S,4R)-
物理化学性质
制备方法
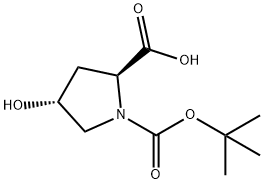
13726-69-7

109384-24-9
以Boc-L-羟脯氨酸为原料合成(2S,4R)-1-Boc-2-氨基甲酰基-4-羟基吡咯烷的一般步骤: 步骤2:制备(2S,4R)-2-氨基甲酰基-4-羟基吡咯烷-1-羧酸叔丁酯 将(2S,4R)-1-(叔丁氧基羰基)-4-羟基吡咯烷-2-羧酸13b(86.4g,0.374mol)溶于1.2L四氢呋喃中,在氩气保护下加入三乙胺(41g,0.411mol)。将反应混合物冷却至-15℃,缓慢加入氯甲酸乙酯(43.84g,0.411mol)。搅拌10分钟后,加入浓氨水(236.8mL)。反应混合物在2小时内缓慢升温至5℃,随后加入氯化铵(32g)。继续搅拌30分钟后,进行相分离。有机相用无水硫酸钠干燥,水相用乙酸乙酯(100mL×2)萃取。合并有机萃取物,减压浓缩,得到(2S,4R)-1-Boc-2-氨基甲酰基-4-羟基吡咯烷(即(2S,4R)-2-氨基甲酰基-4-羟基吡咯烷-1-羧酸叔丁酯13c)(74g,收率86%),为白色固体。
参考文献:
[1] Chemical and Pharmaceutical Bulletin, 2006, vol. 54, # 12, p. 1709 - 1714
[2] Patent: EP2246347, 2010, A1. Location in patent: Page/Page column 39
[3] Patent: WO2003/106456, 2003, A2. Location in patent: Page 28
[4] Bioorganic and Medicinal Chemistry, 2004, vol. 12, # 23, p. 6053 - 6061