7423-55-4
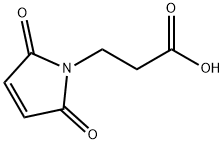 7423-55-4 结构式
7423-55-4 结构式
基本信息
3-马来酰亚胺基丙酸
3-(2,5-DIOXO-2,5-DIHYDRO-PYRROL-1-YL)-PROPIONIC ACID
3-MALEIMIDOPROPIONIC ACID
AKOS BC-0729
BETA-MALEIMIDOPROPIONIC ACID
BMPA
MALEOYL-BETA-ALA-OH
MPA
MPA-OH
N-(2-CARBOXYETHYL)MALEIMIDE
N-BETA-MALEIMIDOPROPIONIC ACID
N-MALEOYL-BETA-ALANINE
RARECHEM AL CD 0912
B-MALEIMIDOPROPIONIC ACID
3-Maleinimidopropionicacid
3-Maleimidopropionic
n-maleoyl-β-alanine
3-MALEIMIDOPROPIONOIC ACID DIHYDRATE
3-(2,5-Dioxo-2,5-dihydro-pyrrol-1-yl)-prop
3-Maleimidopropionic acid, N-(2-Carboxyethyl)maleimide
物理化学性质
制备方法
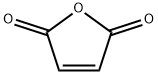
108-31-6
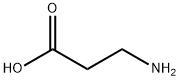
107-95-9

7423-55-4
首先,参照文献方法并进行了适当修改[26,27],以马来酸酐(3.21g,32.78mmol)和β-丙氨酸(3g,33.67mmol)为原料,在冰醋酸(30mL)中形成悬浮液。将该悬浮液加热至回流(油浴温度:170-180℃),持续反应90分钟。反应完成后,将溶液冷却至室温,随后在真空条件下蒸发除去溶剂。为了彻底去除残留的冰醋酸,通过两次与甲苯(2×50mL)共蒸发进行处理。将残余物溶解于水(100mL)和乙酸乙酯(200mL)中,进行液液分离。水层用乙酸乙酯(2×100mL)进一步萃取。合并所有有机相,用无水硫酸钠干燥,随后在真空下浓缩。粗产物通过硅胶柱色谱法纯化,洗脱剂为50%乙酸乙酯的己烷溶液,最终得到3-马来酰亚胺基丙酸(3.7g,21.88mmol,产率67%)。产物的光谱数据与文献报道一致[27]。1H NMR(CDCl3)δ 8.93(宽单峰,1H),6.71(单峰,2H),3.80(三重峰,J = 7.2Hz,2H),3.62-3.66(多重峰,24H),2.67(三重峰,J = 7.2Hz,2H)。
参考文献:
[1] International Journal of Molecular Sciences, 2017, vol. 18, # 9,
[2] Journal of Medicinal Chemistry, 2013, vol. 56, # 24, p. 9955 - 9968
[3] Journal of Controlled Release, 2015, vol. 220, p. 660 - 670
[4] Chemistry - A European Journal, 2015, vol. 21, # 38, p. 13186 - 13190
[5] Journal of the American Chemical Society, 2013, vol. 135, # 11, p. 4333 - 4363
