113118-83-5
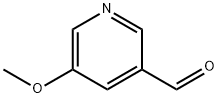 113118-83-5 结构式
113118-83-5 结构式
基本信息
3-甲酰-5-甲氧吡啶
5-甲氧基-3-吡啶甲醛
3-甲氧基-5-醛基吡啶
5-甲氧基-吡啶-3-甲醛
3-FORMYL-5-METHOXYPYRIDINE
3-ForMyl-5-Methoxypyridin...
5-Methoxynicotinaldehyde 97%
5-Methoxypyridin-3-carbaldehyde
5-Methoxy-pyridinecarboxaldehyde
5-Methoxy-3-pyridinecarbaldehyde
5-methxoypyridine-3-carbaldehyde
5-METHOXY-PYRIDINE-3-CARBALDEHYDE
5-Methoxypyridine-3-carboxaldehyde
物理化学性质
制备方法
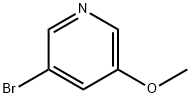
50720-12-2

68-12-2

113118-83-5
将3-溴-5-甲氧基吡啶(100 mg,0.53 mmol)置于装有磁力搅拌棒的烘箱干燥的圆底烧瓶中,并溶解于无水四氢呋喃(1 mL)中。随后,在0℃下加入异丙基氯化镁(0.3 mL),所得混合物于室温下搅拌2小时(溶液变为浅棕色)。然后,缓慢加入N,N-二甲基甲酰胺(0.1 mL)的无水四氢呋喃(0.1 mL)溶液。初始形成的固体逐渐溶解,溶液颜色由浅棕色变为浅黄色。1小时后,将反应混合物冷却至0℃,并用去离子水(2 mL)淬灭反应。分离有机层,水层用二氯甲烷(3×3 mL)进一步萃取。合并有机层,用无水硫酸钠干燥,随后在旋转蒸发器上真空浓缩。粗产物通过梯度硅胶柱色谱法纯化,洗脱剂为己烷与乙酸乙酯的混合溶剂(比例从20:1至1:1),得到目标产物5-甲氧基-吡啶-3-甲醛,为无色浆状物(45 mg,收率63%)。1H NMR(500 MHz,CDCl3):δ 10.09(s,1H),8.65(d,J = 0.9 Hz,1H),8.54(d,J = 3.1 Hz,1H),7.60(dd,J = 5.1,1.5 Hz,1H)。13C NMR(125 MHz,CDCl3):δ 190.6,156.2,145.1,144.8,132.0,116.3,55.7。
参考文献:
[1] Tetrahedron Letters, 2005, vol. 46, # 11, p. 1867 - 1871
[2] Tetrahedron, 2001, vol. 57, # 20, p. 4447 - 4454
[3] Biochemistry, 2010, vol. 49, # 49, p. 10421 - 10439
[4] Patent: US2014/309427, 2014, A1. Location in patent: Paragraph 0157; 0158
[5] Patent: WO2007/84560, 2007, A2. Location in patent: Page/Page column 164
