113209-68-0
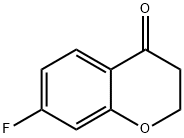 113209-68-0 结构式
113209-68-0 结构式
基本信息
7-氟苯并吡喃-4-酮
7-氟-4-二氢色原酮
7-氟苯并二氢吡喃-4-酮
7-氟-4-苯并二氢吡喃-4-酮
7-FLUOROCHROMAN-4-ONE
7-Fluorochroman-4-one, sum of isomers
7-Fluoro-2,3-dihydro-4H-chromen-4-one
6-fluoro-3,4-dihydro-2H-1-benzopyran-4-one
4H-1-BENZOPYRAN-4-ONE, 7-FLUORO-2,3-DIHYDRO-
7-FluorochroMan-4-one, May contain up to 15% 5-isoMer, 98% (suM of isoMers)
物理化学性质
制备方法
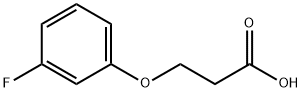
133077-42-6

113209-68-0
以3-(3-氟苯氧基)丙酸为原料合成7-氟苯并二氢吡喃-4-酮的一般步骤如下: C. 7-氟苯并二氢吡喃-4-酮的合成。将3-(3-氟苯氧基)丙酸(2.2 g,0.011 mol)溶于甲苯(25 mL)中,加入亚硫酰氯(4.0 mL,0.054 mol)。将反应混合物加热回流1.5小时,随后在真空下浓缩。将残余物溶解于氯仿(25 mL)中,冷却至-65°C,并逐滴加入三氟甲磺酸(1.5 mL,0.017 mol)。将混合物缓慢升温至室温并继续搅拌2小时。反应完成后,加入水进行分层,有机层用1N NaOH溶液洗涤。合并有机萃取物,用无水硫酸镁干燥,过滤后浓缩,最后通过快速柱色谱法(洗脱剂:己烷/乙酸乙酯)纯化,得到7-氟苯并二氢吡喃-4-酮(0.96 g,收率53%)。 产物表征: HPLC(反相):保留时间RT = 8.22 min。 1H NMR(500 MHz,CDCl3):δ 7.92(dd,J = 8.8, 6.7 Hz,1H),6.75-6.72(m,1H),6.66(dd,J = 9.9, 2.4 Hz,1H),4.55(t,J = 6.4 Hz,2H),2.80(t,J = 6.5 Hz,2H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2016, vol. 59, # 6, p. 2468 - 2477
[2] Patent: US2005/38032, 2005, A1. Location in patent: Page/Page column 30
[3] Patent: WO2006/66746, 2006, A1. Location in patent: Page/Page column 30
[4] Patent: WO2006/66756, 2006, A1. Location in patent: Page/Page column 24-25
[5] Patent: WO2005/92854, 2005, A1. Location in patent: Page/Page column 56
