黄金产品
联系电话:
021-54306202 13764082696
产品介绍:
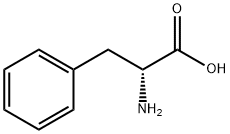
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester hydrochloride
CAS:13033-84-6
纯度:98%
备注:A01635
现货
黄金产品
联系电话:
15387063101
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester hydrochloride
CAS:13033-84-6
纯度:98% HPLC
包装信息:mg;g;kg
备注:所有产品经第三方检测合格后才发货,质量绝对保证!自家实验室,支持定制!专业客服,等您下单!买试剂,找英诺!
库存量:
184g
现货日期:
2026/2/23 11:51:01
黄金产品
联系电话:
021-60345187 13671753212
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
CAS:13033-84-6
纯度:98%
包装信息:100g;500g;1kg;5kg
黄金产品
联系电话:
15161820588; 15161820588
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine Methyl ester hydrochloride
CAS:13033-84-6
纯度:98+
包装信息:1KG;25KG;100KG
大货
现货
联系电话:
022-66211079 13212288319
产品介绍:
英文名称:H-D-Phe-OMe.HCl
CAS:13033-84-6
纯度:98%
备注:1kg
库存量:
1kg
现货日期:
2026/2/25 11:39:49
大货
现货
联系电话:
0514-015380367604 15380367604
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester hydrochloride
CAS:13033-84-6
纯度:99
包装信息:100g 1kg 5kg 10kg 25kg
备注:Kg级|
库存量:
>1g
现货日期:
2026/2/22 9:15:17
大货
现货
联系电话:
158-05183379 15805183379
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester HCL
CAS:13033-84-6
纯度:99%以上 HPLC
包装信息:25kg/纸板桶;100kg;1000kg; 10吨
备注:现货库存销售,GMP工厂,质量保证
库存量:
>1000kg
现货日期:
2026/2/25 9:47:51
现货
联系电话:
027-027-88188016 18062414339
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-phenylalanine methyl ester hydrochloride
CAS:13033-84-6
纯度:99
备注:25公斤每桶
库存量:
>100kg
现货日期:
2026/2/24 10:17:57
现货
联系电话:
15071299552
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:13033-84-6
CAS:13033-84-6
纯度:98%
包装信息:mg;g;kg
备注:自有实验室,可接定制单,质量控制严格,发货必检!专业售前售后客服,确保您购物无忧!
库存量:
96mg
现货日期:
2026/2/23 11:57:08
现货
联系电话:
18171815831
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester hydrochloride
CAS:13033-84-6
纯度:98%
包装信息:5mg,5g
备注:深蓝生物,专注小分子化合物定制和研发,优势产品,常备库存,可定制,可分装,产品均提供最新图谱,合格后付款,质量问题无条件退款,咨询定制咨询!
库存量:
162mg
现货日期:
2026/2/23 11:49:21
现货
联系电话:
17316404525 17316404525
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester hydrochloride
CAS:13033-84-6
纯度:98% HPLC
包装信息:5mg,500mg,1g,100g
备注:欧利希试剂,自有研发团队,产品丰富,图谱齐全,诚信经营,质量保障,质量问题无条件退换,确保您购物无忧!欢迎下单咨询!
库存量:
500mg
现货日期:
2026/2/23 11:50:34
现货
联系电话:
0317-5106596 15533709196
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester hydrochloride
CAS:13033-84-6
纯度:99%化工级 沧州恩科医药科技,大量现货,优势产品,欢迎询价下单,随时为您服务!
包装信息:1元/公斤;2元/公斤;3元/公斤
备注:化工中间体 高纯度现货销售!13033-84-6,D-苯丙氨酸甲酯盐酸盐沧州恩科医药科技有限公司,大量现货,优势产品,欢迎询价下单,随时为您服务!欢迎联系QQ578205830
库存量:
456g
现货日期:
2026/2/24 10:53:05
联系电话:
18210857532; 18210857532
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester hydrochloride, 98%
CAS:13033-84-6
纯度:98%
包装信息:25G;5G
备注:化学试剂、精细化学品、医药中间体、材料中间体
联系电话:
4006356688 18621169109
产品介绍:
中文名称:D-苯甘氨酸甲酯盐酸盐
英文名称:D-Phenylalanine Methyl ester hydrochloride
CAS:13033-84-6
纯度:98%
包装信息:5g;25g
备注:I70060
联系电话:
400-6106006
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐,99%
英文名称:D-Phenylalanine Methyl ester hydrochloride, 99%
CAS:13033-84-6
包装信息:5g
备注:H25920
联系电话:
021-67121386
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine Methyl Ester Hydrochloride
CAS:13033-84-6
纯度:98.0% LC&T
包装信息:25G,5G
备注:试剂级
联系电话:
0757-86329057 18934348241
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine methyl ester hydrochloride
CAS:13033-84-6
纯度:98.00%
包装信息:500g
联系电话:
021-021-58432009 400-005-6266
产品介绍:
中文名称:D-苯丙氨酸甲酯盐酸盐
英文名称:D-Phenylalanine Methyl ester hydrochloride
CAS:13033-84-6
纯度:99%
包装信息:100g,25g,500g,5g
备注:国产化学试剂、精细化学品、医药中间体、材料中间体
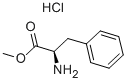 13033-84-6 结构式
13033-84-6 结构式