1395084-25-9
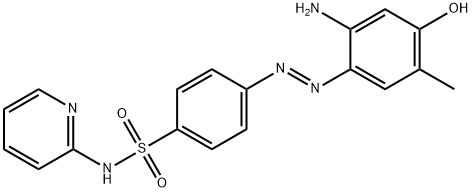 1395084-25-9 结构式
1395084-25-9 结构式
基本信息
4-[(1E)-2-(2-氨基-4-羟基-5-甲基苯基)偶氮]-N-2-吡啶基苯磺酰胺
CS-1882
MS436(95%)
MS 436, >=98%
MS-436
MS 436
MS436 USP/EP/BP
(E)-4-[2-(2-Amino-4-hydroxy-5-methylphenyl)diazenyl]-N-2-pyridinylbenzenesulfonamide
(E)-4-((2-AMINO-4-HYDROXY-5-METHYLPHENYL)DIAZENYL)-N-(PYRIDIN-2-YL)BENZENESULFONAMIDE
4-[(1E)-2-(2-Amino-4-hydroxy-5-methylphenyl)diazenyl]-N-2-pyridinylbenzenesulfonamide
Benzenesulfonamide, 4-[(1E)-2-(2-amino-4-hydroxy-5-methylphenyl)diazenyl]-N-2-pyridinyl-
物理化学性质
制备方法
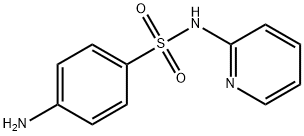
144-83-2
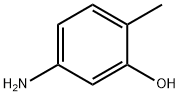
2835-95-2

1395084-25-9
B. (E)-4-((2-氨基-4-羟基-5-甲基苯基)二氮烯基)-N-(吡啶-2-基)苯磺酰胺的合成:向搅拌的4-氨基-N-(吡啶-2-基)苯磺酰胺(12g,0.048mol)在甲醇和乙腈(1:1, 240mL)的混合溶液中缓慢加入浓盐酸(20.4mL),保持反应温度在0℃至-2℃下搅拌5分钟。随后,在惰性气氛保护下,滴加亚硝酸异戊酯(6.48mL,0.553mol),滴加时间控制在10分钟内,滴加完毕后,将反应混合物在0℃下继续搅拌45分钟。同时,制备2-甲基-5-氨基苯酚(5.92g,0.0481mol)和碳酸钾(33.2g,0.24067mol)在水(500mL)中的均匀溶液,通过氮气吹扫15分钟进行脱气处理。脱气完成后,通过套管将该溶液加入到预先制备并保持在0-5℃的重氮盐溶液中,所得反应混合物在0-5℃下搅拌1小时。反应完成后,用1N盐酸调节反应混合物的pH至6,过滤反应混合物。滤液用乙酸乙酯(2×300mL)萃取,合并有机相,减压蒸馏除去溶剂,得到橙红色粗产物。粗产物通过柱色谱法(使用甲醇/二氯甲烷作为洗脱剂,进行两次纯化)纯化,最终得到目标化合物(E)-4-((2-氨基-4-羟基-5-甲基苯基)二氮烯基)-N-(吡啶-2-基)苯磺酰胺(2.6g,收率14%)。TLC条件:5%甲醇/二氯甲烷,Rf值:0.5。HPLC纯度:98.63%,批号:IP 10041325。熔点:217.2℃。质谱:383(M + 1)。1H NMR(500MHz,DMSO-d6)δ:9.22(宽峰,1H),8.0(多重峰,3H),7.9(双峰,2H),7.72(三重峰,1H),7.54(单峰,2H),7.2(双峰,1H),6.8(三重峰,1H),2.21(单峰,6H)。
参考文献:
[1] Patent: WO2012/116170, 2012, A1. Location in patent: Page/Page column 68-69
[2] Journal of Medicinal Chemistry, 2013, vol. 56, # 22, p. 9251 - 9264
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-13959 | (E)-4-((2-AMINO-4-HYDROXY-5-METHYLPHENYL)DIAZENYL)-N-(PYRIDIN-2-YL)BENZENESULFONAMIDE MS436 | 1395084-25-9 | 5mg | 750元 |
| 2025/12/22 | HY-13959 | (E)-4-((2-AMINO-4-HYDROXY-5-METHYLPHENYL)DIAZENYL)-N-(PYRIDIN-2-YL)BENZENESULFONAMIDE MS436 | 1395084-25-9 | 10mM * 1mLin DMSO | 825元 |
| 2025/12/22 | HY-13959 | (E)-4-((2-AMINO-4-HYDROXY-5-METHYLPHENYL)DIAZENYL)-N-(PYRIDIN-2-YL)BENZENESULFONAMIDE MS436 | 1395084-25-9 | 10mg | 1125元 |
常见问题列表
Ki: 30-50 nM (BRD4 bromodomain)
MS436, through a set of water-mediated interactions, exhibits low nanomolar affinity (estimated K i of 30-50 nM) with preference for the first bromodomain over the second. MS436 effectively inhibits BRD4 activity in NF-κB-directed production of NO and pro-inflammatory cytokine interleukin-6 in murine macrophages. MS436 represents a new class of bromodomain inhibitors and will facilitate further investigation of the biological functions of the two bromodomains of BRD4 in gene expression. MS436 exhibits potent affinity of an estimated K i =30-50 nM for the BRD4 BrD1 and a 10-fold selectivity over the BrD2, which is achieved through a unique set of water-mediated intermolecular interactions.