196811-66-2
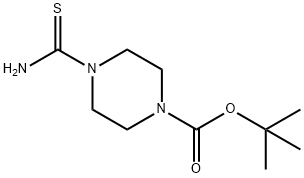 196811-66-2 结构式
196811-66-2 结构式
基本信息
4-(叔丁氧羰基)哌嗪-1-硫代甲酰胺
4-BOC-哌嗪-1-硫酰胺(CAS号:196811-66-2)
1-Boc-4-CarbaMothioylpiperazine
4-(tert-Butoxycarbonyl)piperazine-1-
tert-butyl 4-carbamothioylpiperazine-1-carboxylate
4-(tert-Butoxycarbonyl)piperazine-1-thiocarboxamide
4-THIOCARBAMOYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
4-THIOCARBAMOYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTE...
1-Piperazinecarboxylic acid, 4-(aMinothioxoMethyl)-, 1,1-diMethylethyl ester
物理化学性质
制备方法
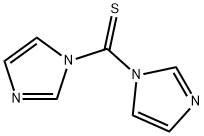
6160-65-2
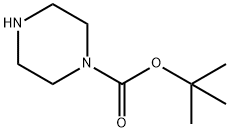
57260-71-6

196811-66-2
在室温下,向N-Boc-哌嗪(5.0 g,26.88 mmol)的无水THF(50 mL)溶液中加入N,N'-硫羰基二咪唑(5.48 g,29.56 mmol),搅拌反应2小时。随后,将反应混合物加热至50℃,持续1小时。反应完成后,将混合物冷却至0℃,并缓慢加入甲醇氨溶液(50 mL,7 N)。将所得混合物在60℃下搅拌20小时。反应结束后,用水稀释反应混合物,并用EtOAc进行萃取。合并有机层,用无水Na2SO4干燥,随后在真空下浓缩。通过快速色谱法对粗产物进行纯化,得到目标化合物4-Boc-哌嗪-1-硫酰胺。产量:4.0 g(92%收率),为白色固体。1H NMR(400 MHz,DMSO-d6)δ:9.2(m,2H),3.16-3.14(m,2H),2.49-2.48(m,6H),1.30(s,9H)。LCMS(方法A):246.2(M + H),保留时间2.93分钟,纯度95.3%(最大)。
参考文献:
[1] Patent: WO2016/30443, 2016, A1. Location in patent: Page/Page column 106
[2] Patent: WO2017/144637, 2017, A1. Location in patent: Page/Page column 39
[3] Patent: US2010/197703, 2010, A1. Location in patent: Page/Page column 77
[4] Patent: WO2015/48662, 2015, A2. Location in patent: Page/Page column 105
[5] Bioorganic and Medicinal Chemistry, 2008, vol. 16, # 4, p. 1613 - 1631
