19932-85-5
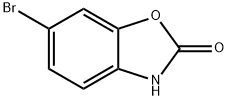 19932-85-5 结构式
19932-85-5 结构式
基本信息
6-溴-苯并噁唑酮
6-溴-2-苯并唑酮
6-溴苯并恶唑-2-酮
6-溴-2-苯并噁唑酮
6-溴-2-苯并恶唑啉酮
6-溴-1,3-苯并噁唑酮
6-溴-2(3H)-苯并唑酮
6-溴-2(3H)-苯并恶唑
6-溴-2(3H)-苯并恶唑酮
6-BROMOBENZOXAZOLINE
6-Bromobenzoxazolone
6-Bromo-2-benzoxazolone
6-BROMO-BENZOXAZOLINONE
6-broMobenzoxazol-2-one
6-Bromo-2-benzoxazolinone
6-Bromobenzoxazol-2(3H)-one
6-BROMO-2(3H)-BENZOXAZOLONE
6-Bromo-3H-benzoxazol-2-one
物理化学性质
制备方法
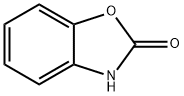
59-49-4

19932-85-5
以2-苯并噁唑啉酮为原料合成6-溴苯并[d]恶唑-2(3H)-酮的一般步骤:将3H-1,3-苯并恶唑-2-酮(5.00g,37.00mmol)溶于乙酸(50mL)中,缓慢滴加溴(1.9mL,37.0mmol)。反应混合物于20℃下搅拌反应4小时。反应完成后,将混合物倒入冰水中,过滤收集沉淀,用水洗涤,空气干燥,得到粉红色固体产物(7.48g,34.8mmol,收率94%)。产物熔点为191.6-192.3℃。1H NMR(300MHz,DMSO-d6):δ11.81(s,1H),7.57(dd,J=1.9Hz,0.3Hz,1H),7.30(dd,J=8.3Hz,1.9Hz,1H),7.04(dd,J=8.3Hz,0.3Hz,1H)。13C NMR(75MHz,DMSO-d6):δ154.5,144.5,130.3,126.9,113.5,113.2,111.7。LCMS m/z计算值[M-H]+:211.9,213.9;实测值:211.8,213.8。
参考文献:
[1] European Journal of Medicinal Chemistry, 2018, vol. 159, p. 104 - 125
[2] Patent: US6372933, 2002, B1. Location in patent: Page column 5 - 6
[3] Patent: US2008/138413, 2008, A1. Location in patent: Page/Page column 22
[4] Monatshefte fur Chemie, 2011, vol. 142, # 1, p. 67 - 80
[5] Patent: EP1251128, 2002, A1. Location in patent: Page 38
