2309-07-1
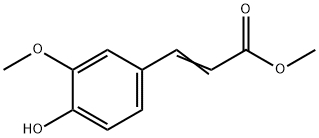 2309-07-1 结构式
2309-07-1 结构式
基本信息
4-羟基-3-甲氧基肉桂酸甲酯
FERULIC ACID METHYL ESTER
METHYL 3-(4-HYDROXY-3-METHOXY)CINNAMATE
METHYL 4-HYDROXY-3-METHOXYCINNAMATE
METHYL FERULATE
METHYL TRANS-3-(4'-HYDROXY-3'-METHOXYPHENYL)-ACRYLATE
3-(4-hydroxy-3-methoxyphenyl)-2-propenoicacimethylester
4-hydroxy-3-methoxy-cinnamicacimethylester
3-(3-Methoxy-4-hydroxyphenyl)acrylic acid methyl ester
3-(4-Hydroxy-3-methoxyphenyl)propenoic acid methyl ester
3-Methoxy-4-hydroxybenzeneacrylic acid methyl ester
Maritin F
Fumalic acid methyl ester
物理化学性质
制备方法

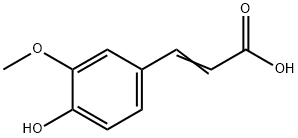
67-56-1
1135-24-6
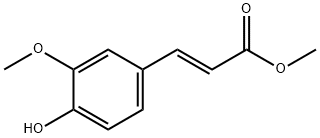
22329-76-6
以甲醇和3-甲氧基-4-羟基苯基丙烯酸为原料合成(E)-3-(4-羟基-3-甲氧基苯基)丙烯酸甲酯的一般步骤:将阿魏酸(1.94 g,10 mmol)溶解于无水甲醇(3.20 g,100 mmol)中,加入0.25 mL浓硫酸作为催化剂。在搅拌下回流反应,通过薄层色谱(TLC)监测反应进程。待原料点消失后,停止反应并冷却至室温。将反应混合物倒入冰水体系中,用10%过量的碳酸钠溶液中和剩余的酸。随后,用饱和盐水洗涤反应混合物,并用乙醚进行萃取。分离有机相后,合并有机层并用无水硫酸钠干燥。过滤除去干燥剂后,减压蒸除溶剂得到粗产物。粗产物用正己烷洗脱,进一步通过重结晶纯化,得到白色固体状的(E)-3-(4-羟基-3-甲氧基苯基)丙烯酸甲酯,质量为1.42 g(理论质量为2.08 g),收率为68.3%。
参考文献:
[1] European Journal of Medicinal Chemistry, 2009, vol. 44, # 1, p. 332 - 344
[2] Chinese Chemical Letters, 2014, vol. 25, # 3, p. 474 - 478
[3] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 19, p. 6085 - 6088
[4] Molecular Crystals and Liquid Crystals, 2016, vol. 624, # 1, p. 59 - 68
[5] Organic and Biomolecular Chemistry, 2009, vol. 7, # 11, p. 2367 - 2377
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-W018643 | 阿魏酸甲酯 Ferulic acid methyl ester | 2309-07-1 | 500 mg | 250元 |
| 2025/12/22 | HY-W018643 | 阿魏酸甲酯 Ferulic acid methyl ester | 2309-07-1 | 10mM * 1mLin DMSO | 280元 |
| 2025/12/22 | HY-W018643 | 阿魏酸甲酯 Ferulic acid methyl ester | 2309-07-1 | 1 g | 400元 |
常见问题列表
|
p38
|
Ferulic acid methyl ester (25 µg/mL) has no cytotoxic effects on BMDMs after treatment for 6 h, 18 h, 48 h.
Ferulic acid methyl ester (Methyl ferulate; 5, 10, 25 µg/mL) suppresses TNFα, IL6, IFNγ but not IL10, inhibits NO generation at 10 and 25 µg/mL, in primary bone marrow derived-macrophages (BMDMs).
Ferulic acid methyl ester (25 µg/mL) inhibits COX-2 expression, blocks p-p38 and p-JNK.
Cell Viability Assay
| Cell Line: | Primary bone marrow derived-macrophages (BMDMs) |
| Concentration: | 25 µg/mL |
| Incubation Time: | 6 h, 18 h, 48 h |
| Result: | Showed no cytotoxic effects on BMDMs. |
Western Blot Analysis
| Cell Line: | RAW 246.7 cells |
| Concentration: | 10 µg/mL and 25 µg/mL |
| Incubation Time: | For 1 h before stimulation with LPS |
| Result: | Significantly suppressed COX-2 expression at 25 µg/mL. |
