2420-16-8
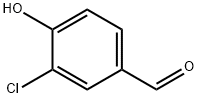 2420-16-8 结构式
2420-16-8 结构式
基本信息
-氯-4-羟基苯甲醛
3-氯-4-羟基苯甲醛, 99+%
3-CHLORO-4-HYDROXYBENZALDEHYDE
AKOS B028967
TIMTEC-BB SBB004014
TIMTEC-BB SBB005887
Benzaldehyde, 3-chloro-4-hydroxy-
3-CHLORO-4-HYROXYBENZALDEHYDE
3-Chloro-4-hydroxybenzaldehyde 98%
3-Chloro-4-hydroxybenzaldehyde, 99+%
物理化学性质
安全数据
Eye Dam. 1
Skin Irrit. 2
Skin Sens. 1
STOT SE 3
制备方法
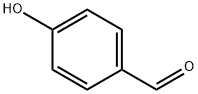
123-08-0

2420-16-8
以4-羟基苯甲醛为原料合成3-氯-4-羟基苯甲醛的一般步骤如下:将N-氯代琥珀酰亚胺(1.1g,8.18mmol)加入到4-羟基苯甲醛(1.0g,8.18mmol)溶解于10ml氯仿的溶液中。将反应混合物在50℃下加热搅拌15小时。反应完成后,冷却至室温,减压浓缩除去溶剂。将残余物溶解于25ml乙酸乙酯中,依次用水和饱和食盐水洗涤,有机相用无水硫酸钠干燥。过滤后,减压浓缩得到粗产物。粗产物通过硅胶柱色谱纯化,以12%乙酸乙酯的己烷溶液为洗脱剂,最终得到1.1g(产率86%)的3-氯-4-羟基苯甲醛。1H-NMR(400MHz,CDCl3)δ(ppm):9.840(s,1H),7.898-7.894(d,1H,J = 1.6Hz),7.749-7.724(dd,1H,J = 8.4Hz),7.164-7.143(d,1H,J = 8.4Hz),6.288(s,1H)。
参考文献:
[1] Tetrahedron Letters, 1994, vol. 35, # 28, p. 5043 - 5046
[2] Patent: WO2010/127212, 2010, A1. Location in patent: Page/Page column 35
[3] Patent: WO2010/127208, 2010, A1. Location in patent: Page/Page column 55
[4] Tetrahedron Asymmetry, 1997, vol. 8, # 24, p. 4003 - 4006
[5] Synthetic Communications, 2012, vol. 42, # 24, p. 3655 - 3663,9

