25535-16-4
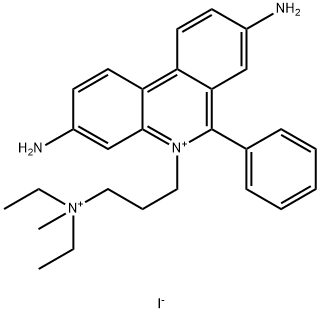 25535-16-4 结构式
25535-16-4 结构式
基本信息
碘化酶
3,8-DIAMINO-5-(3-DIETHYL-AMINOPROPYL)-6-PHENYLPHENANTHRIDIUM IODIDE METHIODIDE
3,8-DIAMINO-5-(3-[DIETHYLMETHYLAMMONIO])-6-PHENYLPHENAN-THRIDINIUM DIIODIDE
3,8-DIAMINO-5-[3-(DIETHYLMETHYLAMMONIO)PROPYL]-6-PHENYLPHENANTHRIDINIUM
3,8-DIAMINO-5-[3-(DIETHYLMETHYLAMMONIO)PROPYL]-6-PHENYLPHENANTHRIDINIUM DIIODIDE
PHENANTHRIDINIUM, 3,8-DIAMINO-5-[3-(DIETHYLMETHYLAMMONIO)PROPYL]-6-PHENYL-, DIIODIDE
PI
PROPIDIUM IODIDE
3,8-diamino-5-(3-(diethylmethylammonio)propyl)-6-phenyl-phenanthridiniudii
3,8-diamino-5-(3-(diethylmethylammonio)propyl)-6-phenylphenanthridiniumdiiod
3,8-diamino-5-(3-diethylaminopropyl)-6-phenylphenanthridiniumiodidemethiodi
Propidium iodide solution
Propidium diiodide
PROPIDIUM IODIDE 95-98%
PROPIDIUM IODIDE, FOR FLUORESCENCE
PROPIDIUM IODIDE 95%
-Cellstain-PiSolution
-Cellstain-Pi
PROPIDIUM IODIDE RED POWDER
3,8-Diamino-5-[3-(diethylmethylamino)propyl]-6-phenylphenanthridium,diiodide
物理化学性质
ex/em : 535/617 nm (和DNA)
消光系数(ε) : 5400 cm−1M−1 (水/DNA)
安全数据
应用领域
常见问题列表
碘化丙啶是一种可对DNA染色的细胞核染色试剂,常用于细胞凋亡检测,英文全称是Propidium Iodide。它是一种溴化乙啶的类似物,在嵌入双链DNA后释放红色荧光。
碘化丙啶本身荧光很弱,在没有DNA存在的水溶液中,碘化丙啶只有很弱的493nm的激发峰和636nm的发射峰,但当加入DNA后,碘化丙啶嵌入DNA双链,并荧光强度增加20-30倍,同时激发峰红移到535nm,发射峰蓝移到617nm。
尽管碘化丙啶的消光系数较低,但是它的斯托克斯位移很大,可让同时检测碘化丙啶荧光和荧光素类红色荧光,常常用于流式细胞实验中检测细胞凋亡,细胞周期等。碘化丙啶也可以用于荧光显微镜,共聚焦显微镜等中的细胞核染色实验。
Propidium Iodide is a cell-membrane impermeable dye with characteristic excitation maximum at 535 nm and emission maximum at 617 nm which intercalates with nucleic acids with a stoichiometry of one dye per 4-5 base pairs with little sequence preference. Propidium Iodide has evidenced of having no toxic effects on neurons, being today’s most common marker for membrane integrity and cell viability when applied prior to fixation (pre-fixation Propidium Iodide staining method). The pre-fixation staining has been widely used for quantitative assessments of neuronal cell decline in models of acute neurodegeneration, visualized as intensely labeled PI + -pycnotic nuclei of degenerating neurons . Propidium Iodide cannot cross the membrane of live cells, making it useful to measure the percentage of apoptotic cells by flow-cytometric analysis. The flow cytometric data shows an excellent correlation with the results obtained with both electrophoretic and colorimetric methods. This new rapid, simple and reproducible method proves useful for assessing apoptosis of specific cell populations in heterogeneous tissues such as bone marrow, thymus and lymph nodes.
