308846-06-2
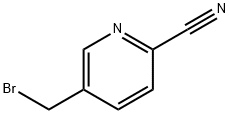 308846-06-2 结构式
308846-06-2 结构式
基本信息
2-氰基-5-溴甲基吡啶
2-氰基-5-溴乙基吡啶
5-溴甲基-2-睛基吡啶
5-溴甲基-2-氰基吡啶
5-(溴甲基)吡啶-2-甲腈
2-氰基-5-溴甲基吡啶 10G
5-(bromomethyl)picolinonitrile
2-cyano-5-broMide-Methyl pyridine
5-(broMoMethyl)-2-pyridinecarbonitrile
5-(BROMOMETHYL)PYRIDINE-2-CARBONITRILE
2-Pyridinecarbonitrile, 5-(broMoMethyl)-
2-Cyano-5-bromomethylpyridine ISO 9001:2015 REACH
5-(Bromomethyl)-2-cyanopyridine, 5-(Bromomethyl)picolinonitrile
物理化学性质
制备方法
1620-77-5

308846-06-2
以2-氰基-5-甲基吡啶为起始原料合成5-(溴甲基)-2-氰基吡啶的一般步骤:将市售的2-氰基-5-甲基吡啶(1.90 g,16.1 mmol)溶解于氯仿(100 mL)中,随后加入N-溴代琥珀酰亚胺(4.29 g,24.1 mmol)和α,α-偶氮二异丁腈(0.792 g,4.82 mmol)。将反应混合物在60℃下搅拌6小时。反应完成后,将混合物冰浴冷却,过滤分离沉淀物,滤液经减压浓缩。通过硅胶柱色谱法纯化所得残余物,得到目标化合物5-(溴甲基)-2-氰基吡啶(1.69 g,产率53%)。1H NMR(CDCl3)δ(ppm):4.80(s,2H),8.03(dd,J = 0.92, 8.07 Hz,1H),8.12(dd,J = 2.20, 8.07 Hz,1H),8.81(d,J = 1.83 Hz,1H)。
参考文献:
[1] Patent: EP2163554, 2010, A1. Location in patent: Page/Page column 52-53
[2] Patent: WO2007/28083, 2007, A2. Location in patent: Page/Page column 69-70
[3] Bioorganic and Medicinal Chemistry Letters, 2002, vol. 12, # 7, p. 1017 - 1022
[4] Journal of Medicinal Chemistry, 2003, vol. 46, # 17, p. 3612 - 3622
[5] Patent: WO2011/20615, 2011, A1. Location in patent: Page/Page column 57; 58
