35150-07-3
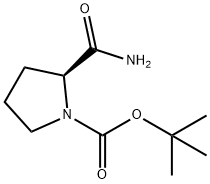 35150-07-3 结构式
35150-07-3 结构式
基本信息
N-BOC-L-脯氨酰胺
BOC-L-PROLINAMIDE
BOC-L-PROLINE AMIDE
BOC-L-PRO-NH2
BOC-PRO-NH2
BOC-PYRD(2)-NH2
L-1-N-BOC-PROLIDINAMIDE
(L)-1-N-BOC-PROLINAMIDE
N-ALPHA-T-BUTOXYCARBONYL-L-PROLINE AMIDE
N-ALPHA-T-BUTOXYCARBONYL-L-PYRROLIDINE-2-CARBOXYLIC ACID AMIDE
N-ALPHA-T-BUTYLOXYCARBONYL-L-PROLINE AMIDE
N-ALPHA-TERT-BUTYLOXYCARBONYL-L-PROLINE AMIDE
N-TERT-BUTOXYCARBONYL-L-PROLINE AMIDE
N-(TERT-BUTOXYCARBONYL)PROLINE AMIDE
(S)-2-(AMINOCARBONYL)-1-PYRROLIDINECARBOXYLIC ACID, 1,1-DIMETHYLETHYL ESTER
(S)-2-CARBAMOYL-PYRROLIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
DL-1-BOC-PROLINAMIDE
Boc-D-Prolinamide
D-1-N-Boc-prolinamide
tert-Butyl (2R)-2-carbamoylpyrrolidine-1-carboxylate
物理化学性质
制备方法
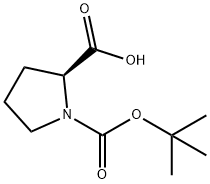
15761-39-4

35150-07-3
通用方法:将BOC-L-脯氨酸(1.00g,4.65mmol)、二碳酸二叔丁酯(1.52g,6.97mmol)、碳酸氢铵(0.55g,6.97mmol)和吡啶(1.0mL)溶于二恶烷(20mL)中,室温搅拌反应6小时。反应完成后,用二氯甲烷萃取产物,依次用1M盐酸和饱和氯化钠溶液洗涤有机相,无水硫酸钠干燥,过滤并减压浓缩。向浓缩物中加入正己烷(100mL),超声处理促使产物以白色固体形式析出,得到N-叔丁氧羰基-L-脯氨酰胺(0.85g,收率85%)。产物经1H NMR(300MHz,CDCl3)表征:δ4.37-4.34(m,1H),3.47-3.36(m,2H),2.07-1.85(m,4H),1.49(s,9H)。质谱(ESI)m/z 215 [M + H]+。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2002, vol. 10, # 6, p. 1719 - 1729
[2] Canadian Journal of Chemistry, 2007, vol. 85, # 2, p. 85 - 95
[3] Journal of Agricultural and Food Chemistry, 2012, vol. 60, # 35, p. 8544 - 8551
[4] Journal of Medicinal Chemistry, 2017, vol. 60, # 1, p. 228 - 247
[5] Bioorganic and Medicinal Chemistry, 2013, vol. 21, # 23, p. 7418 - 7429
常见问题列表
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW027013872605 | N-叔丁氧羰基-d-脯氨酰胺 (R)-tert-Butyl 2-carbamoylpyrrolidine-1-carboxylate | 35150-07-3 | 100g | 1208元 |
| 2025/12/22 | B3756 | N-叔丁氧羰基-L-脯氨酰胺 N-(tert-Butoxycarbonyl)-L-prolinamide | 35150-07-3 | 1g | 30元 |
| 2025/12/22 | XW027013872604 | N-叔丁氧羰基-d-脯氨酰胺 (R)-tert-Butyl 2-carbamoylpyrrolidine-1-carboxylate | 35150-07-3 | 25g | 303元 |
