4743-17-3
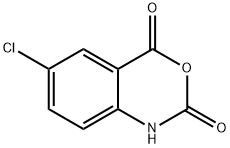 4743-17-3 结构式
4743-17-3 结构式
基本信息
6-氯-4H-3,1-苯并恶嗪-2,4(1H)-二酮
5-CHLOROISATONIC ANHYDRIDE
6-CHLORO-1,2-DIHYDRO-4 H-3,1-BENZOXAZINE-2,4-DIONE
6-CHLORO-1H-BENZO[D][1,3]OXAZINE-2,4-DIONE
6-chloro-4h-3,1-benzoxazine-2,4(1h)-dione
AKOS BBS-00002892
TIMTEC-BB SBB003570
5-Chloroisotoic anhydride
6-Chloro-2H-3,1-benzoxazine-2,4(1H)-dione
5-Chloroisatoic
5-Chloroisatoic anhydride 97%
5-Chloroisatoic anyhydride
1,4-Dihydro-6-chloro-2H-3,1-benzoxazine-2,4-dione
6-Chloro-1H-3,1-benzoxazine-2,4-dione
6-Chloro-4H-3,1-benzoxazine-2(1H),4-dione
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
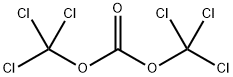
32315-10-9
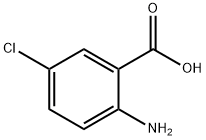
635-21-2

4743-17-3
步骤1 6-氯-1H-苯并[d][1,3]噁嗪-2,4-二酮的合成:向500 mL三颈圆底烧瓶中加入2-氨基-5-氯苯甲酸(17 g,0.1 mol)和1,2-二氯乙烷(200 mL)。在80℃下,向上述溶液中缓慢滴加三光气(21 g,0.21 mol)在1,2-二氯乙烷(100 mL)中的溶液。滴加完毕后,将反应混合物在80℃下继续加热搅拌3小时。反应完成后,将混合物置于冰水浴中冷却至室温。通过过滤收集析出的白色固体沉淀,并用适当的溶剂洗涤,干燥后得到6-氯-1H-苯并[d][1,3]噁嗪-2,4-二酮(19 g,收率97%)。产物经1H NMR(300 MHz,DMSO-d6)表征:δ 11.85(br,1H),7.88(d,J = 2.4 Hz,1H),7.78(dd,J = 8.7, 2.4 Hz,1H),7.15(d,J = 8.7 Hz,1H)。
参考文献:
[1] Patent: US2010/120741, 2010, A1. Location in patent: Page/Page column 78
[2] Patent: WO2011/112731, 2011, A2. Location in patent: Page/Page column 174
[3] Journal of Medicinal Chemistry, 2013, vol. 56, # 16, p. 6434 - 6456
[4] Chemical Biology and Drug Design, 2010, vol. 75, # 5, p. 444 - 454
[5] Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 10, p. 2295 - 2299
