57102-42-8
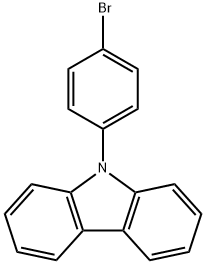 57102-42-8 结构式
57102-42-8 结构式
基本信息
9-(4-溴苯基)咔唑
4-溴-9(H)苯基咔唑
9-(4-溴苯基)咔唑,98%
9-(4-溴苯基)-9H-咔唑
9-(4-溴苯基)咔唑 100G
N-(4-溴苯基)咔唑(C18H12BRN)
9-(4-溴苯基)咔唑9-(4-溴苯基)-9H-咔唑
9-(4-溴苯基)-9H-咔唑 9-(4-BROMOPHENYL)-9H-CARBAZOLE
9-(4-Bromophenyl)carbazole
9-(4-BroMophenyl)carbazole90%
9-(4-Bromophenyl)carbazole >
N-(4-BroMophenyl)-9H-carbazole
9H-Carbazole,9-(4-broMophenyl)-
9-(4-Bromophenyl)carbazole
(9-(4-BROMOPHENYL))-9H-CARBAZOLE
9-(4-broMophenyl)-9H-carbazole 4BPC
(9-(4-BROMOPHENYL))-9H-CARBAZOLE ISO 9001:2015 REACH
物理化学性质
制备方法
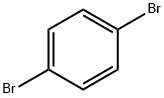
106-37-6
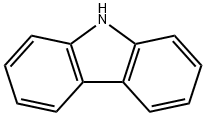
86-74-8

57102-42-8
步骤2:4-(咔唑-9-基)二苯胺(缩写:YGA)的合成;在步骤2中,根据下面所示的(i)和(ii)合成YGA。 (i)9-(4-溴苯基)咔唑的合成;首先,将56g(240mmol)1,4-二溴苯,31g(180mmol)咔唑,4.6g(24mmol)碘化铜,66g(480mmol)碳酸钾和2.1g(8mmol)18-冠-6-醚加入300mL三颈烧瓶中,并用氮气置换烧瓶中的空气。随后,向烧瓶中加入8mL N,N-二甲基丙烯脲(缩写:DMPU),并将反应混合物在180℃下搅拌6小时。反应完成后,将混合物冷却至室温,通过抽滤去除沉淀。滤液依次用稀盐酸、饱和碳酸氢钠水溶液和饱和氯化钠水溶液洗涤,然后用无水硫酸镁干燥。干燥后,将溶液自然过滤并浓缩,通过硅胶柱色谱法(洗脱剂:己烷/乙酸乙酯=9:1)纯化得到的油状物,最后用氯仿和己烷进行重结晶。最终,得到21g目标产物9-(4-溴苯基)咔唑的浅棕色板状晶体,产率为35%。
参考文献:
[1] Chemistry Letters, 2008, vol. 37, # 9, p. 986 - 987
[2] Patent: CN106883163, 2017, A. Location in patent: Paragraph 0030; 0031; 0032; 0033
[3] New Journal of Chemistry, 2010, vol. 34, # 7, p. 1317 - 1322
[4] Patent: CN105585577, 2016, A. Location in patent: Paragraph 0220; 0221; 0222; 0223; 0224
[5] Patent: EP2292618, 2011, A1. Location in patent: Page/Page column 17
