502161-03-7
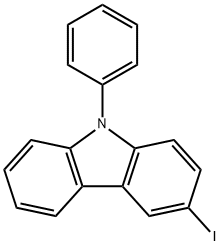 502161-03-7 结构式
502161-03-7 结构式
基本信息
3-碘-N-苯基咔唑
N-苯基-3-碘咔唑
3-碘代-9-苯基咔唑
3-碘-9-苯基-9H-咔唑
3-碘-N-苯基咔唑(C18H12IN)
3-碘-9苯基咔唑(3-碘代-9-苯基咔唑)
3-碘-9苯基咔唑(CAS号:502161-03-7)
3-碘-9苯基咔唑3-IODO-N-PHENYLCARBAZOLE
3-Iodo-N-phenylcarba
3-iodo-N-phenylcarbazol
3-Iodo-9-phenylcarbazole
3-Iodo-N-phenylcarbazole
3-Iodo-9-phenylcarbazole>
3-iodo-9-phenyl-9H-carbazole
3- iodine-9- phenylcarbazole
9H-Carbazole, 3-iodo-9-phenyl-
3-Iodo-N-Phenylcarbazole (IPC)
物理化学性质
制备方法
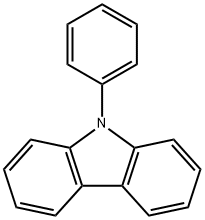
1150-62-5

502161-03-7
以N-苯基咔唑为原料合成3-碘-9苯基咔唑的一般步骤:将2.433 g(10 mmol)中间体化合物A(N-苯基咔唑)溶解于100 mL 80%乙酸溶液中,随后依次加入1.357 g(5.35 mmol)碘单质(I2)和0.333 g(1.46 mmol)邻-正辛酸(H5IO6)。在氮气保护下,将反应混合物于80℃搅拌反应2小时。反应完成后,用乙醚(50 mL)对反应混合物进行三次萃取。合并有机层,用无水硫酸镁干燥,随后减压蒸发去除溶剂。所得粗产物通过硅胶柱色谱法纯化,得到3.23 g中间体化合物B(3-碘-9苯基咔唑),为白色固体,收率为87%。1H NMR(CDCl3, 300 MHz)δ(ppm):8.43(d, 1H),8.05(d, 1H),7.62(dd, 1H),7.61-7.75(m, 2H),7.51-7.43(m, 3H),7.41-7.35(m, 2H),7.27(dd, 1H),7.14(d, 1H)。
参考文献:
[1] Patent: EP1661888, 2006, A1. Location in patent: Page/Page column 11
[2] Patent: US2008/174237, 2008, A1. Location in patent: Page/Page column 13-14
[3] Patent: EP2149555, 2010, A1. Location in patent: Page/Page column 19
[4] Patent: KR101791161, 2017, B1. Location in patent: Paragraph 0116; 0123-0128
[5] Patent: EP2738166, 2014, A1. Location in patent: Paragraph 0162
