57561-39-4
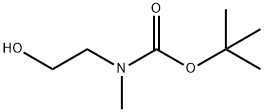 57561-39-4 结构式
57561-39-4 结构式
基本信息
BOC-N-甲基氨基乙醇
BOC-N-ME-乙醇胺
N-BOC-N-甲基乙醇胺
N-BOC-N-甲基氨基乙醇
N-BOC-N-甲氧氨基乙醇
N-BOC-N-甲基氨基乙醇 1G
(2-羟基乙基)甲基氨基甲酸叔丁酯
2-羟基乙基(甲基)氨基甲酸叔丁酯
N-BOC-N-甲基乙醇胺,95%
N-Boc-N-meth
BOC,ME-N-ET-OH
Boc,Me-Glycinol
Boc-N-Me-Gly-OL
BOC-N-ME-AMINOETHANOL
N-BOC-N-METHYL-ETHANOLAMINE
N-Boc-N-methyl-aminoethanol
N-Methyl N-Boc-ethanolaMine
2-(N-BOC-METHYLAMINO)ETHANOL
物理化学性质
制备方法

109-83-1

24424-99-5

57561-39-4
一般步骤:向2-(甲基氨基)乙醇(500 mg,0.53 mL,6.66 mmol)的二氯甲烷(20 mL)溶液中加入二碳酸二叔丁酯(1.48 g,6.79 mmol),室温搅拌反应1小时。反应完成后,用饱和氯化钠水溶液和二氯甲烷萃取反应混合物。合并有机层,用无水硫酸镁干燥,过滤。滤液经减压浓缩,得到N-BOC-N-甲基氨基乙醇(无色油状物,定量收率)。产物表征数据如下:1H NMR(200 MHz,CDCl3)δ 3.74(q,J = 10.5, 5.2 Hz,2H),3.25(t,J = 5.2 Hz,2H),2.91(s,3H),1.45(s,9H);质谱m/z(相对强度)144(20),102(24),57(70),44(100)。
参考文献:
[1] Journal of Organic Chemistry, 2000, vol. 65, # 20, p. 6368 - 6380
[2] Patent: US2009/42872, 2009, A1. Location in patent: Page/Page column 19
[3] Patent: US2007/4675, 2007, A1. Location in patent: Page/Page column 92-93
[4] Patent: WO2007/81091, 2007, A1. Location in patent: Page/Page column 26
[5] Patent: WO2011/5028, 2011, A2. Location in patent: Page/Page column 11
