611-08-5
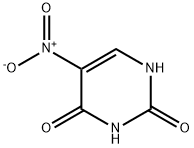 611-08-5 结构式
611-08-5 结构式
基本信息
5-硝基脲嘧啶
2,4-二羟基-5-硝基嘧啶
5-硝尿嘧啶
5-硝基尿嘧啶, 98+%
2,4-DIHYDROXY-5-NITROPYRIMIDINE
5-NITRO-2,4-DIHYDROXYPYRIMIDINE
5-NITRO-2,4-PYRIMIDINEDIOL
5-NITROPYRIMIDINE-2,4(1H,3H)-DIONE
5-NITROPYRIMIDINE-2,4-DIOL
5-NITROURACIL
NITROURACIL
2,4-Dihydroxy-5-nitropyrimidin
3h)-pyrimidinedione,5-nitro-4(1h
5-Nitro-1H-pyrimidine-2,4-dione
5-Nitro-2,4(1H,3H)-pyrimidindion
5-nitrouracil(2,4-dihydroxy-5-nitropyrimidine)
5-Nitrouracil,99%
5-NITROURACIL/PRAZOSIN DOXAZOSIN
5-nitro-4(1h,3h)-pyrimidinedione
5-NITRO-PYRIMIDIN-2,4-DIOL
5-NITROURACIL, 98+%
5-NITROURACIL extrapure
Uracil, 5-nitro-
物理化学性质
安全数据
制备方法
66-22-8

611-08-5
以嘧啶-2,4(1H,3H)-二酮为原料合成5-硝基嘧啶-2,4(1H,3H)-二酮的一般步骤:在温度不超过50℃的条件下,将硝酸溶液(5.34 mL,120 mmol,70%溶液)逐滴加入浓硫酸(19.7 mL,360 mmol,98%溶液)中。随后,将嘧啶-2,4(1H,3H)-二酮(7.204 g,60 mmol)分批加入搅拌的混合酸溶液中,同时控制反应温度不超过50℃。反应混合物加热至55℃并维持3小时,之后冷却至室温以下,用冰水(38 mL)淬灭反应。通过过滤收集生成的白色沉淀,用少量冰水洗涤,并在55℃下减压干燥,得到白色固体5-硝基嘧啶-2,4(1H,3H)-二酮(8.658 g,55.11 mmol,收率92%)。产物性质:分解温度>288℃;薄层色谱Rf值0.00(展开剂比例为1:10的乙酸乙酯/己烷);红外光谱(ZnSe池,固体)vmax:3142-2811 cm^-1(中,宽,OH/NH/CH),1732 cm^-1(强,C=O),1677和1624 cm^-1(强,NO2),1417 cm^-1(中,CH),1356 cm^-1(中,NO2),1318 cm^-1(强,芳香族R2NH/R3N),1234 cm^-1(强,-OH),975和832 cm^-1(弱,芳香环弯曲);1H NMR(500 MHz,d6-DMSO):δ8.84(1H,单峰,6-CH)ppm;13C NMR(125 MHz,d6-DMSO):δ155.5(4-C=O),149.8(6-CH),147.9(2-C=O),125.1(5-CNO2)ppm。
参考文献:
[1] European Journal of Medicinal Chemistry, 2015, vol. 95, p. 29 - 34
[2] American Chemical Journal, 1905, vol. 33, p. 443
[3] American Chemical Journal, 1908, vol. 40, p. 31
[4] Journal of Biological Chemistry, 1908, vol. 4, p. 410
[5] Journal of the American Chemical Society, 1919, vol. 41, p. 786
知名试剂公司产品信息
5-Nitrouracil, 99+%(611-08-5)
5-Nitrouracil,>99.0%(LC)(T)(611-08-5)
