773-76-2
中文名称
5,7-二氯-8-羟基喹啉
英文名称
5,7-Dichloro-8-hydroxyquinoline
CAS
773-76-2
EINECS 编号
212-258-3
分子式
C9H5Cl2NO
MDL 编号
MFCD00006786
分子量
214.05
MOL 文件
773-76-2.mol
更新日期
2026/02/24 10:07:05
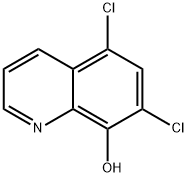 773-76-2 结构式
773-76-2 结构式
基本信息
中文别名
5,7-二氯-8-羟基喹啉5,7-二氯-8-羧基喹啉
5,7-二氯-8-羟基氮杂萘
[克]氯辛
英文别名
5,7-DICHLORO-8-HYDROXYQUINOLINE5,7-DICHLORO-8-OXINE
5,7-DICHLORO-8-QUINOLINOL
AKOS BBS-00002684
CHLOROXINE
5,7-Dichlor-8-hydroxychinolin
5,7-Dichloro-8-hydroquinoline
5,7-Dichloro-8-hydroxyquinolin
5,7-Dichloro-8-oxychinolin
5,7-Dichloro-8-oxyquinoline
5,7-dichloro-8-quinolino
5,7-Dichlorooxine
5,7-Dichloroxine
8-Quinolinol,5,7-dichloro-
Capitrol
Chhoroox-ine
Chlofucid
CHQ
Clofuzid
component of Capitrol Cream Shampoo
所属类别
医药中间体:喹啉类化合物物理化学性质
外观性状无色或淡黄色针状结晶。熔点180-181℃。溶于苯、丙酮、醚、二硫化碳、乙醇和浓硫酸,不溶于水,易溶于氢氧化钠、氢氧化钾呈黄色溶液。
熔点178-180 °C(lit.)
沸点305.47°C (rough estimate)
密度1.3320 (rough estimate)
折射率1.5500 (estimate)
储存条件Sealed in dry,Room Temperature
溶解度Chloroform (Slightly), DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Heated, Sonicated)
酸度系数(pKa)2.30±0.30(Predicted)
形态蓬松粉末
颜色浅米色
Merck14,2175
BRN153606
稳定性Light Sensitive
InChI1S/C9H5Cl2NO/c10-6-4-7(11)9(13)8-5(6)2-1-3-12-8/h1-4,13H
InChIKeyWDFKMLRRRCGAKS-UHFFFAOYSA-N
SMILESOc1c(Cl)cc(Cl)c2cccnc12
NIST化学物质信息8-Quinolinol, 5,7-dichloro-(773-76-2)
安全数据
警示词危险
危险性描述H302-H315-H318-H335
危险品标志Xn,Xi
WGK Germany3
RTECS号VC5350000
TSCAYes
海关编码29334900
存储类别11 - Combustible Solids
危险性类别Acute Tox. 4 Oral
Eye Dam. 1
Skin Irrit. 2
STOT SE 3
Eye Dam. 1
Skin Irrit. 2
STOT SE 3
制备方法
方法一
由8-羟基喹啉经氯化而得。将10g8-羟基喹啉溶于100g冰醋酸中,然后通过氯气直至溶液成为金黄色为止。将反应物注入1升水中,即析出5g7-二氯-8-羟基喹啉,过滤后用200ml丙酮重结晶而得成品。5,7-二氯-8-羟基喹啉价格(试剂级)
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-B0295 | 5,7-二氯-8-羟基喹啉 Chloroxine | 773-76-2 | 100mg | 100元 |
| 2025/12/22 | HY-B0295 | 5,7-二氯-8-羟基喹啉 Chloroxine | 773-76-2 | 1 g | 200元 |
| 2025/12/22 | HY-B0295 | 5,7-二氯-8-羟基喹啉 Chloroxine | 773-76-2 | 5 g | 270元 |
常见问题列表
生物活性
Chloroxine是一种人工合成的抗菌化合物,加入洗发液中,有效治疗头皮屑和脂溢性皮炎。靶点
Bacterial
体外研究
By using the Jouyban-Acree model, the Chloroxine solubility is well correlated obtaining RAD values lower than 3.64% and RMSD values lower than 8.82 × 10 −6 . Chloroxine is preferentially solvated by water for the studied mixtures in water-rich compositions; while within intermediate and co-solvent-rich compositions, Chloroxine is preferentially solvated by DMSO (DMF, NMP or 1,4-dioxane) in DMSO (DMF, NMP or 1,4-dioxane) + water mixtures. It is shown that the change in solvent-solvent interaction energy accounted by cavity term governed the solubility variation of Chloroxine in all aqueous mixtures.

