79517-01-4
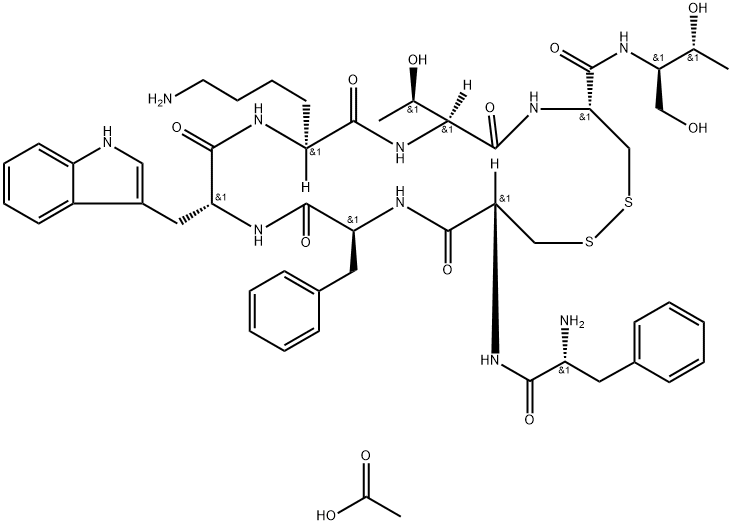 79517-01-4 结构式
79517-01-4 结构式
基本信息
SMS-201-995
SANDOSTATIN
SMS 201-995ac
Sandostatin LAR
r*))-(racetate(salt)
Octreotide (SMS 201-995)
M.W. 1019.24 C49H66N10O10S2
OCTREOTIDE (SMS 201-995), ACETATE
FCFWKTCT-OL (DISULFIDE BRIDGE: 2-7)
物理化学性质
安全数据
常见问题列表
本品的化学结构上的第1、 4、8个氨基酸为非天然氨基酸,故不易受酶破坏,半衰期亦较长。皮下注射本品吸收迅速而完全,血药浓度达峰值时间为0.5小时,分布容积为0.27L/kg,半衰期为1.5小时,代谢清除率为160ml/min。静注时呈双相消除,半衰期α相和β相分别为10 分钟和90分钟。血浆蛋白结合率为65%。
临床上奥曲肽主要用于治疗门脉高压引起的食管静脉曲张出虫、肢端巨大症、消化道出血如肝硬化、食管静脉曲张出血、消化性溃疡出血和应激性溃疡出血,胰腺疾病如重型急性胰腺炎、胰损伤或手术后胰腺瘘,以及预防胰腺手术后并发症。还用于胃肠道瘘管,消化系内分泌肿瘤如肠血管活性肽瘤、胃泌素瘤、胰高血糖素瘤、异位ACTH综合征、类癌综合征,肢端肥大症,突眼性甲亢症,全结肠切除后出现的持续性、顽固性腹泻和艾滋病相关性腹泻。鞘内注射可治疗癌性疼痛。
(2)治疗门脉高压引起的食管静脉曲张出血:0.1mg静脉注射, 以后0.5mg,每2小时1次静脉滴注。
(3)应激性溃疡及消化道出血:0.1mg皮下注射,每日3次。
(4)重型胰腺炎:0.1mg皮下注射,每日4次,疗程3~7天;胰损伤或手术后胰瘘,皮下注射,每次0.1mg,每8小时1次,连用7~14日。
(5)胃肠道瘘管和消化道内分泌系统肿瘤的辅助治疗:皮下注射0.1mg,每日3次,疗程10~14天。
(6)消化系系内分泌肿瘤、突眼性甲状腺肿、肢端肥大症及艾滋病相关性腹泻:皮下注射0.1mg,每日3次,肢端肥大症疗程10~14天。用后主要有注射部位疼痛或针刺感,一般于15分钟缓解。
消化道不良反应有厌食、恶心、呕吐、腹泻、腹部痉挛疼痛等,偶见高血糖、胆石、糖耐量异常和肝功能异常(γ-谷氨酰转移酶增高及转氨酶轻度增高)等。肾、胰腺功能异常和胆石症患者慎用。孕妇、哺乳期妇女和儿童禁用。
Octreotide-treated groups show a significant reduction in the tumor volume when compared with saline group. Octreotide-PPSG (1.4 mg/kg, i.p.) shows greater antitumor effect than Octreotide-soln (100 μg/kg, i.p.). Octreotide-treatments results in significant inhibitory effect on the expression levels of SSTR2 and SSTR5 in primary HCC-bearing rats compared with the saline group. Octreotide-PPSG appears to inhibit the expression of SSTR2 and SSTR5 to a greater extent than that of Octreotide-soln treated group. A test dose of octreotide acetate significantly decreases the serum gastrin level to approximately one third of the baseline in 2 hr and the effect lasted approximately for 6 hr. On day 21, treatment with sustained-release formulation of octreotide acetatea (5 mg intramuscular, q 4 wk) is initiated.
