84000-11-3
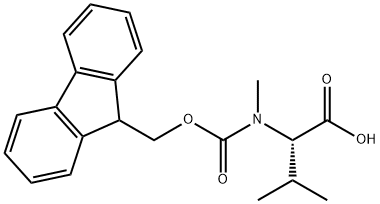 84000-11-3 结构式
84000-11-3 结构式
基本信息
Fmoc-N-甲基-L-缬氨酸
N-芴甲氧羰基-N-甲基-L-缬氨酸
FMOC-MEVAL-OH
FMOC-N-ALPHA-METHYL-L-VALINE
FMOC-N-ME-L-VAL-OH
FMOC-N-METHYL-L-VALINE
FMOC-N-ME-VALINE
FMOC-N-ME-VAL-OH
N-ALPHA(9-FLUORENYLMETHOXYCARBONYL)-N-ALPHA-METHYL-L-VALINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-N-ALPHA-METHYL-L-VALINE
N-ALPHA-FMOC-N-ALPHA-METHYL-L-VALINE
FMOC-N-ME-VAL 98.5+%
N-(9-Fluorenylmethyloxycarbonyl)-N-methyl-L-valine
物理化学性质
制备方法
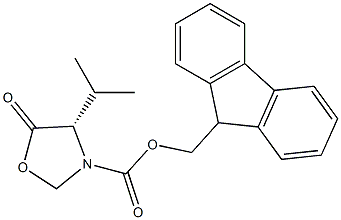
84000-01-1
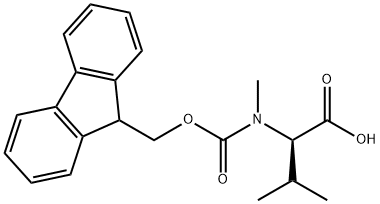
103478-58-6
以(S)-3-Fmoc-4-异丙基-5-氧代噁唑烷为原料合成(R)-2-((((9H-芴-9-基)甲氧基)羰基(甲基)氨基)-3-甲基丁酸的一般步骤如下:将Fmoc-缬氨酰恶唑烷酮(2.80 g,8.00 mmol)溶解于氯仿(40 mL)中。向该溶液中依次加入三氟乙酸(1.85 mL,24.0当量)和三乙基硅烷(3.83 mL,24.0当量)。将反应混合物于室温下搅拌,直至反应完全(24-72小时)。反应完成后,将溶液浓缩,并于乙醚和5%碳酸氢钠溶液之间分配。合并水相,用5M盐酸调节pH至2,随后用乙酸乙酯萃取。合并有机相,用无水硫酸镁干燥,浓缩后得到Fmoc-N-甲基缬氨酸(4),为白色固体(2.35 g,收率83%)。Fmoc-N-甲基缬氨酸通过乙酸乙酯重结晶,得到白色针状晶体:熔点186-187℃(文献值33:185-187℃);低分辨质谱(ESI)m/z [M + H]+ 354.1(100%),[M + Na]+ 376.1(61%);红外光谱(NaCl)νmax(cm-1)3420(宽,OH),2964(脂肪族CH),1700(C=O),1445,1305(C=C);1H NMR(300 MHz,CDCl3)δH(ppm)7.63(2H,d,J=7.5 Hz,ArH),7.46(2H,d,J=6.9 Hz,ArH),7.26(2H,t,J=7.2 Hz,ArH),7.17(2H,t,J=7.5 Hz,ArH),4.28(2H,m,CHCH2O),4.22(1H,m,(Ar)2CHCH2),4.13(1H,m,NCHCO),2.79(3H,d,J=4.2 Hz,NCH3),2.02(1H,m,CH3CHCH3),0.88(3H,dd,J=6.6,19.2 Hz,CH3CHCH3),0.72(3H,dd,J=6.6,13.8 Hz,CH3CHCH3)(红外和1H NMR数据与文献报道一致);13C NMR(75 MHz,CDCl3)δC(ppm)175.5,159.6,146.6,143.9,130.4,129.7,127.7,122.6,70.3,66.9,49.9,33.1,30.2,22.5,21.7。
参考文献:
[1] Journal of Organic Chemistry, 2005, vol. 70, # 17, p. 6918 - 6920
[2] European Journal of Organic Chemistry, 2013, # 21, p. 4509 - 4513
[3] Tetrahedron, 2014, vol. 70, # 14, p. 2351 - 2358