845306-08-3
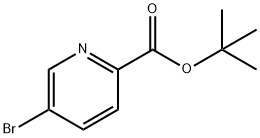 845306-08-3 结构式
845306-08-3 结构式
基本信息
5-溴吡啶-2-羧酸叔丁酯
5-溴吡啶-2-甲酸叔丁酯
5-溴吡啶-2-甲酸叔丁醇酯
5-Bromopicolinic acid tert-butyl ester
TERT-BUTYL 5-BROMOPYRIDINE-2-CARBOXYLATE 98
tert-Butyl 5-bromopyridine-2-carboxylate 98%
5-Bromopyridine-2-carboxylic acid t-butyl ester
5-Bromo-pyridine-2-carboxylic acid tert-butyl ester
5-Bromo-2-pyridinecarboxylic acid 1,1-dimethylethyl ester
2-Pyridinecarboxylic acid, 5-broMo-, 1,1-diMethylethyl ester
TERT-BUTYL 5-BROMOPYRIDINE-2-CARBOXYLATE 98 ISO 9001:2015 REACH
物理化学性质
制备方法
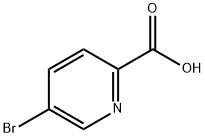
30766-11-1

75-65-0

845306-08-3
以2-羧酸-5-溴吡啶和叔丁醇为原料合成5-溴吡啶-2-甲酸叔丁酯的一般步骤如下:将2-羧酸-5-溴吡啶(118mg,0.58mmol)与吡啶(0.3mL,0.39mmol)和叔丁醇(1mL)混合,随后加入对甲苯磺酰氯(262mg,1.38mmol)。将反应混合物在40℃下搅拌10分钟,然后在室温下继续搅拌2小时。反应完成后,加入饱和碳酸氢钠溶液(4mL)并搅拌5分钟。接着加入二乙醚,将两相混合物搅拌10分钟。分离有机层,用盐水洗涤,用无水硫酸镁干燥,随后在减压下浓缩。通过硅胶柱色谱法纯化残余物,洗脱剂为戊烷:乙酸乙酯(100:0至80:20),得到5-溴吡啶-2-甲酸叔丁酯,为无色固体,收率73%(110mg)。产物经1H-NMR(CDCl3,400MHz)确认:δ 1.65(s,9H),7.95(m,2H),8.88(m,1H)。质谱(ES+)显示m/z 539 [M2Na]+。
参考文献:
[1] Patent: EP1595881, 2005, A1. Location in patent: Page/Page column 62
[2] Patent: WO2005/14571, 2005, A1. Location in patent: Page/Page column 27
[3] Patent: WO2006/29906, 2006, A1. Location in patent: Page/Page column 16-17
[4] Patent: WO2012/143599, 2012, A1. Location in patent: Page/Page column 47-48

