88043-21-4
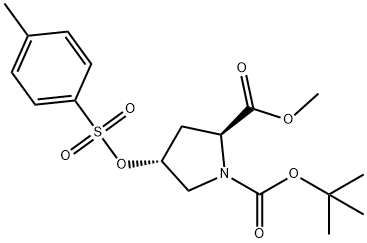 88043-21-4 结构式
88043-21-4 结构式
基本信息
BOC-反式-4-对甲苯磺酰氧基-L-脯氨酸甲酯
N-BOC-反式-4-对甲苯磺酰氧基-L-脯氨酸甲酯
N-叔丁氧羰基-反-4-对甲苯磺酰氧基-L-脯氨酸甲酯
N-叔丁氧羰基-反式-4-对甲苯磺酰氧基-L-脯氨酸甲酯
(2S,4R)-N-(叔丁氧羰基)-4-对甲苯磺酰基羟脯氨酸甲酯
N-叔丁氧羰基-反-4-对甲苯磺酰氧基-L-脯氨酸甲酯
(2S,4R)-4-{[(4-甲基苯基)磺酰基]氧基]-1,2-吡咯烷二羧酸 1-(1,1-二甲基乙基) 2-甲酯
(4R)-4-TsO-N-Boc-Pro-OMe
Boc-trans-4-tosyloxy-L-proline methyl ester
N-BOC-TRANS-4-TOSYLOXY-L-PROLINE METHYL ESTER
N-Boc-trans-4-(p-tosyloxy)-L-proline methyl ester
trans-N-tert-Butyloxycarbonyl-4-tosyloxy-L-proline Methyl Ester
N-tert-Butoxycarbonyl-O-p-toluenesulfonyl-L-proline methyl ester
N-Boc-trans-4-(p-toluenesulfonyloxy)-L-proline Methyl ester, 98%
N-TERT-BUTYLOXYCARBONYL-O-P-TOLUENESULFONYL-L-PROLINE METHYL ESTER
BOC-trans-4-tosyloxy-L-proline methyl ester:(4R)-4-TsO-N-Boc-Pro-OMe
物理化学性质
制备方法
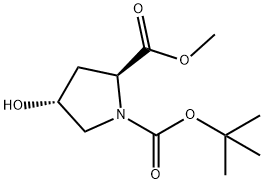
74844-91-0

98-59-9

88043-21-4
以N-Boc-反式-4-羟基-L-脯氨酸甲酯和对甲苯磺酰氯为原料合成BOC-反式-4-对甲苯磺酰-L-脯氨酸甲酯的一般步骤:将1-叔丁基-2-甲基-(2S,4R)-4-羟基吡咯烷-1,2-二羧酸酯(30.00g,122.3mmol)和三乙胺(51.1mL,367mmol)溶于CH2Cl2(500mL)中,随后加入对甲苯磺酰氯(28.0g,147mmol)和4-二甲基氨基吡啶(1.00g,8.18mmol)。反应混合物在室温下搅拌过夜。TLC(50%乙酸乙酯的己烷溶液)显示反应未完全,因此补加0.5当量(14g)的对甲苯磺酰氯,继续搅拌过夜。TLC确认原料完全消耗后,反应混合物用300mL CH2Cl2稀释,依次用1.0N HCl(2×100mL)、1.0N NaOH(2×100mL)和盐水(1×100mL)洗涤,有机层用Na2SO4干燥。减压浓缩溶剂,得到含有结晶固体的深色油状物。通过硅胶柱色谱(450g硅胶,25%乙酸乙酯的己烷溶液)纯化,得到47.4g(97%)浅棕色油状产物。1H NMR(400MHz,CDCl3)δ ppm 1.32-1.48(m,9H),2.07-2.23(m,1H),2.34-2.62(m,4H),3.52-3.66(m,2H),3.72(s,3H),4.28-4.46(m,1H),4.96-5.12(m,1H),7.33-7.41(m,2H),7.79(d,J=8.29Hz,2H);MS ES+ m/z 300.3(M-99)和344.3(M-55)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2004, vol. 14, # 24, p. 6107 - 6111
[2] Patent: WO2009/137130, 2009, A2. Location in patent: Page/Page column 182
[3] Patent: WO2018/26792, 2018, A1. Location in patent: Page/Page column 68
[4] Patent: US2008/306086, 2008, A1. Location in patent: Page/Page column 5-6; 15-16
[5] Bulletin of the Korean Chemical Society, 2016, vol. 37, # 8, p. 1259 - 1264
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | B5247 | N-叔丁氧羰基-反-4-对甲苯磺酰氧基-L-脯氨酸甲酯 N-(tert-Butoxycarbonyl)-trans-4-(p-toluenesulfonyloxy)-L-proline Methyl Ester | 88043-21-4 | 200mg | 95元 |
| 2025/12/22 | B5247 | N-叔丁氧羰基-反-4-对甲苯磺酰氧基-L-脯氨酸甲酯 N-(tert-Butoxycarbonyl)-trans-4-(p-toluenesulfonyloxy)-L-proline Methyl Ester | 88043-21-4 | 1g | 200元 |
| 2025/12/22 | XW028804321402 | N-Boc-反式-4-对甲苯磺酰氧基-L-脯氨酸甲酯 Boc-trans-4-Tosyloxy-L-proline methyl ester | 88043-21-4 | 5g | 261元 |
